Chapter 8
RECAP 8.1
Exergonic reactions release free energy because the energy of the reactants is greater than that of the products. The reverse is true for endergonic reactions, which require an input of energy. ΔG is the free energy change of a reaction—
products minus reactants. A positive ΔG means that the reaction is endergonic and requires energy, while a negative ΔG indicates that a reaction is exergonic and releases energy. Endergonic reactions require the input of energy to create more ordered molecules. The second law of thermodynamics states that order tends to increase in the universe. Endergonic reactions are coupled in time and space with exergonic reactions, which increase the disorder and release the energy needed for the endergonic reactions to proceed. Overall, organisms need to take in energy from their environment continually to maintain these reactions.
RECAP 8.2
ATP has terminal phosphate groups that repel one another, so it takes energy to form a bond between them. Some of this energy is stored as potential energy. Also, hydrolysis of phosphate releases energy because the free energy of the P~O bond is higher than the free energy of the OH bond formed by hydrolysis.
The reaction is endergonic (positive ΔG).
ATP hydrolysis can drive the reaction (ΔG = −7.3 kcal/mol).
RECAP 8.3
Enzymes have a three-
dimensional (tertiary) structure with an active site in which the substrate fits. Chemical groups at the active site also bind to the substrate non- covalently. While an enzyme-
catalyzed reaction proceeds more rapidly to its equilibrium than an uncatalyzed reaction, the actual equilibrium point is unaffected. The presence of water may prevent O2 from reaching the enzyme.
Boiling denatures proteins, so polyphenol oxidase is irreversibly altered by boiling, and its active site is destroyed.
RECAP 8.4
As part of the catalysis, an enzyme can add or remove H+ in a substrate, which may facilitate reaction with the substrate. The course of these H+ reactions involves acidic or basic R groups on amino acids at the active site. Metal ions of enzymes may gain or lose electrons during the reaction, donating them temporarily to the substrate.
Coenzymes are small and nonprotein; enzymes are large proteins or RNA. Coenzymes do not catalyze reactions; enzymes are catalysts. Coenzymes add or remove chemical groups (e.g., H+) and are permanently changed as a result of the reaction; enzymes are not permanently changed by participating in a reaction.
At the beginning, the reaction rate will increase linearly with added enzyme since the enzyme will be saturated with substrate even after many additions. Thus the reaction rate will increase proportionally with the amount of enzyme added, but only until the enzyme is no longer saturated with substrate. At this point the enzyme will be present in excess with respect to substrate, and additional enzyme will have no additional effect. The curve will level off to a constant maximum rate when this point is reached.
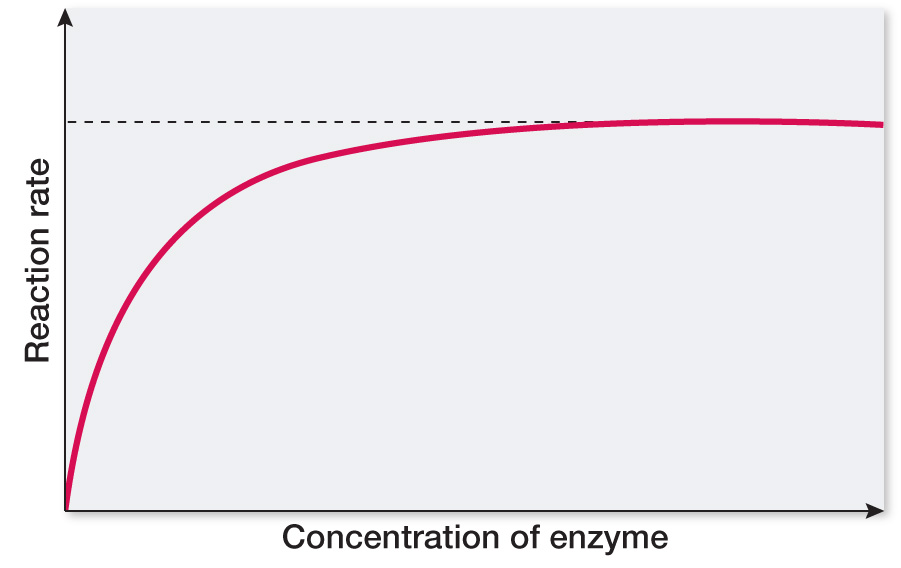
RECAP 8.5
Feedback inhibition occurs in a biochemical pathway when that pathway’s end product can act as an inhibitor of the enzyme that catalyzes the first step in the pathway. In a systems diagram, each node represents an enzymatic transformation. Feedback inhibition can cross multiple pathways, thereby allowing the changing concentration of one molecule to affect several pathways that lead to its synthesis.
See Figure 8.17. A competitive inhibitor binds to the active site of the enzyme and shifts the equilibrium to enzyme molecules in the active form.
To determine whether catalase has an allosteric or a nonallosteric mechanism, perform an experiment with varying amounts of substrate and plot the rate of catalase-
versus- substrate concentration. An S- shaped curve will indicate an allosteric mechanism. A hyperbolic curve will indicate a nonallosteric enzyme. To determine if a pollutant is a competitive or a noncompetitive inhibitor, add the pollutant to the catalase to lower the rate of reaction, then add increasing amounts of substrate. A competitive inhibitor will be removed from the active site and the rate of reaction will increase. A noncompetitive inhibitor will not allow the rate to increase as more substrate is added.
WORK WITH THE DATA, P. 165
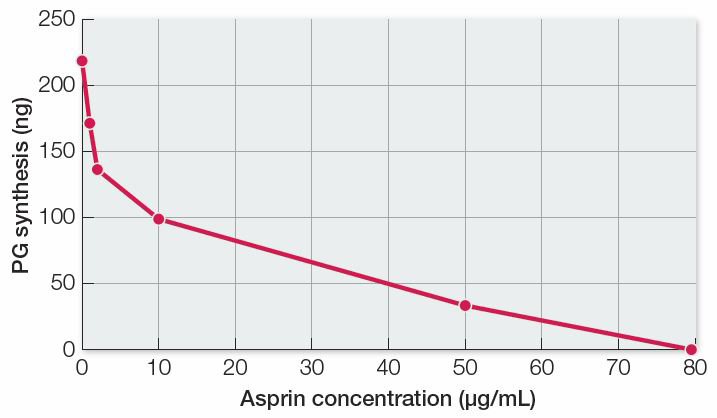
There is an inverse relationship between enzyme activity (PG synthesis) and aspirin concentration, so aspirin is an inhibitor of the reaction in lung tissue homogenates. Because aspirin also inhibited PG synthesis in human platelets, we can conclude that inhibition occurs in different tissues of different organisms.
In all three individuals, aspirin given in vivo inhibited PG synthesis by platelets. This generalizes the results from the test tube experiment to a living organism.
FIGURE QUESTIONS
Figure 8.7 The synthesis of ATP has a ΔG of +7.3 kcal/mol. So any reaction driving this synthesis must have a ΔG of at least –7.3 kcal/mol. Because of the second law of thermodynamics, the transfer of energy is not efficient (entropy increases), so the ΔG of the driving reaction would be more than +7.3 kcal/mol.
Figure 8.8 Yes. In this case, activation energy is needed to make the substrate(s) able to take up energy and then the reaction proceeds.
Figure 8.12 No. Typically, the tertiary structure of a protein is due to weak noncovalent forces such as hydrophobic interactions, ionic attractions, and hydrogen bonds. Binding of substrate alters these forces but not covalent bonds.
Figure 8.19 Lysosomal enzymes have an amino acid sequence and therefore tertiary structure that exposes their active site at pH 4.8. At pH 7.2, the tertiary structure does not expose the active site, so the enzyme is inactive.
APPLY WHAT YOU’VE LEARNED
The molecules are luciferin, oxygen, ATP, and magnesium ion. Luciferin reacts with oxygen to undergo chemical change that involves emission of light as this change takes place. In order to drive this reaction, phosphate bonds in ATP are hydrolyzed, which releases chemical energy. Some of this energy is then released in the form of light as luciferin and oxygen react. Magnesium ion is needed as a cofactor of the enzyme that catalyzes reactions involving the other molecules.
The firefly takes in food as a source of chemical energy. As the food is digested, some of the chemical energy in the bonds of the food molecules is stored in phosphate bonds of ATP as ADP is phosphorylated to form ATP. This chemical energy is released when ATP is hydrolyzed during the luciferin reaction, with some of the energy released in the form of light.
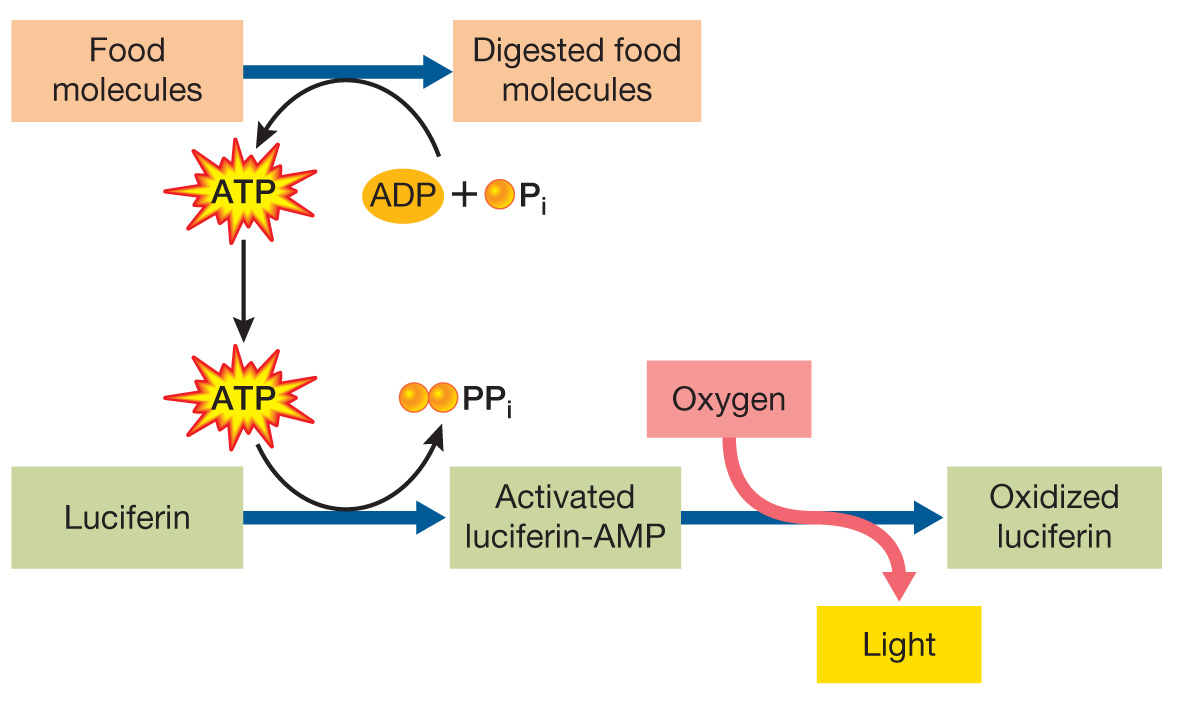
The enzyme’s catalytic activity is abolished when the enzyme is heated. The catalytic activity is also affected by changes in pH. The effect of heating is likely due to disruption of the enzyme’s tertiary structure by high heat; most proteins cannot maintain their active shape if they are exposed to high heat. The effect of changing pH is likely due to changes in ionization of basic amino acid side chains of lysine and arginine or acidic side chains of aspartic acid and glutamic acid. Changes in ionization in these side chains can affect binding of the substrate or interactions between groups within the enzyme that stabilize its three-
dimensional structure. This is an example of induced fit, which the enzyme uses to orient substrates with one another and with amino acid side chains of the enzyme that are important in its catalytic mechanism. This shape change of the enzyme excludes water from the active site, ensuring that ATP will not be hydrolyzed by water and will react only with luciferin to transfer AMP and release pyrophosphate, PPi.