 2Na(s) + Cl2(g) → 2NaCl(s)Solid sodium reacts with chlorine gas to produce solid sodium chloride. 2Na(s) + Cl2(g) → 2NaCl(s)Solid sodium reacts with chlorine gas to produce solid sodium chloride. |  CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)Methane gas and oxygen gas burn to produce carbon dioxide gas and water. CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)Methane gas and oxygen gas burn to produce carbon dioxide gas and water. | 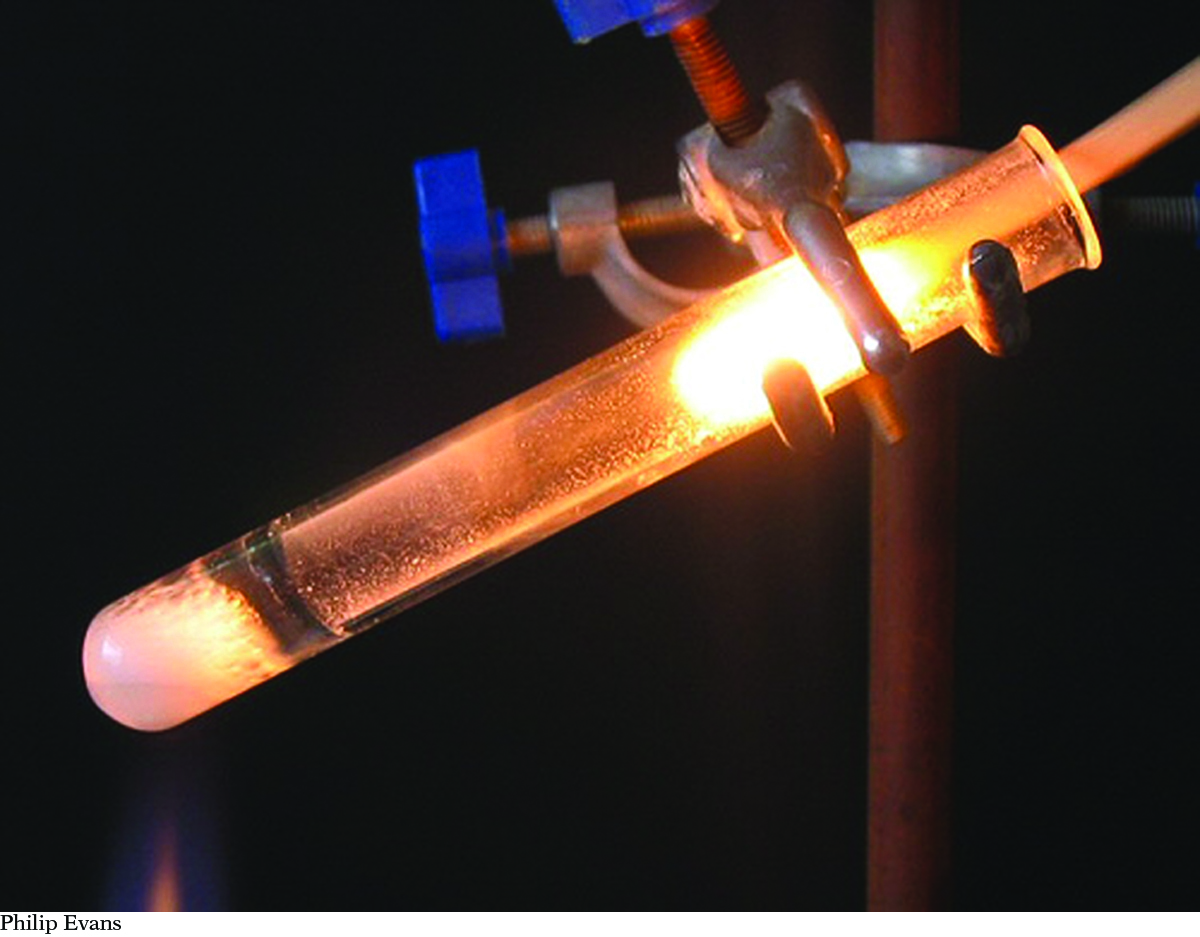 2KClO3(s) → 2KCl(s) + 3O2(g)Solid potassium chlorate is heated to form solid potassium chloride and oxygen gas. 2KClO3(s) → 2KCl(s) + 3O2(g)Solid potassium chlorate is heated to form solid potassium chloride and oxygen gas. |





