Scientific Notation
The mass of a hydrogen atom is 0.0000000000000000000000014 g. The number of atoms in 1 mol of carbon is 602,000,000,000,000,000,000,000. To make these numbers easier to read, compare, and use in calculations, scientists use scientific notation. The long numbers above can be written as 1.4 × 10224 and 6.02 × 1023.
Each number is written as a decimal with one digit to the left of the decimal point times a power of 10. A number written in scientific notation has the form a × 10n, where 1 ≤ a < 10 or –10 < a ≤1, and n is an integer. In other words, if a is a positive number, it is greater than or equal to 1 and less than 10. If a is a negative number, it is less than or equal to –1 and greater than –10.
Use the properties of exponents as you combine numbers written in scientific notation.
For addition and subtraction, convert all the numbers so that the powers of 10 you are combining are the same. (The converted numbers might not be in scientific notation.)
For multiplication, add the exponents. For any values of b, m, and n,
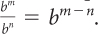
For division, subtract the exponents. For any nonzero value of b, and any values of m and n, .
Example 1
Billions
Write these numbers in scientific notation.
2,110,000,000
0.0074
Solution
Move the decimal point so that there is only one digit to the left of it, in this case 2. The decimal point moved nine places. The number in scientific notation is 2.11 × 109. This means that to get the number in standard notation you start with 2.11 and move the decimal nine places to the right.
Move the decimal after the 7 to get 7.4. From here, the decimal will need to move four places to the left, so the number in scientific notation is 7.4 × 1024.
Example 2
Operations with Scientific Notation
Calculate these values.
(3.2 × 10 5) (4.0 × 1028)
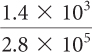
(3.2 × 10 5) + (4.0 × 104)
Solutions
1.28 × 1022. Multiplying the decimals gives 12.8. The product of the powers of 10 is 1023. To put this into scientific notation, divide 12.8 by 10; to keep things balanced, increase the power of 10 by a factor of 10 to –2.
5.0 × 107. The division of the numbers gives 0.50. To divide the powers of 10, subtract the exponents: 3 – (– 5), or 8. To rewrite in scientific notation, multiply 0.50 by 10, so subtract 1 from the power of 10.
3.6 × 10 5. Change the second number to 0.4 × 10 5before you add.
Practice Exercises
Use scientific notation to write the speed of light, 299,792,4 58 meters per second, accurate to five digits.
How many zeros would follow the final 2 if 6.022 × 1023 were written without scientific notation?
Calculate these values.
(3.0 × 1014)(4.0 3 1024)
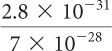
(1.21 × 1024)(4.18 × 104)
(3.61 × 107) – (2.5× 106)
Answers
| 1. 2.9979 × 108 | 2. 20 | ||
| 3a. 1.2 × 1011 | 3b. (4 × 1024) | 3c. 5.06 × 100, or 5.06 | 3d. (3.36 × 107) |