Chemical Names and Formulas
Recall from Unit 1: Alchemy that all matter is made up of elements that can combine with one another to form compounds. Chemists have created an organized system of chemical names and formulas that specify what elements are present in a given compound. In Unit 2: Smells, chemical names and formulas were used to predict the smell of different substances. So knowing the chemical name and formula of a compound can often be useful in predicting properties of substances. Understanding some basic patterns in naming and writing formulas is also useful because it allows chemists from different parts of the world to communicate chemical information easily. Examine the table that contains binary ionic compounds and binary covalent compounds.
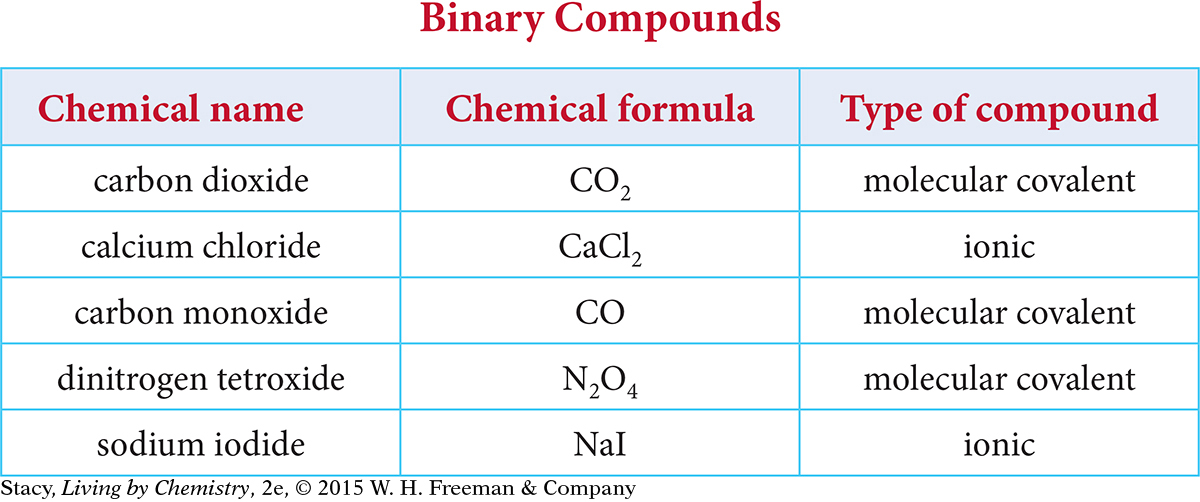
Here are some patterns you might notice.
Each compound is made up of two different elements.
The name of each compound ends in the suffix -ide.
The names of the molecular covalent compounds include prefixes: di-, mono-, tri-, and tetr- correspond to the number of atoms of each element in the formula.
The names of the ionic compounds do not include prefixes.
The compounds in the table are called binary compounds because they are made up of only two different elements. Binary ionic compounds are made up of metal and nonmetal atoms and binary covalent compounds are made up of nonmetals only. Notice that with binary ionic compounds, the name alone does not tell you how many atoms of each element are in the compound. However, with binary covalent compounds, the prefixes in the name indicate how many of each atom are present in the formula.
Be careful not to confuse binary compounds with elements that exist as diatomic molecules such as hydrogen, H2, nitrogen, N2, and oxygen, O2. These diatomic molecules are made up of only one type of element. The elements that exist in nature as diatomic molecules include hydrogen, nitrogen, oxygen, and the halogens.
NAMES OF BINARY COVALENT COMPOUNDS
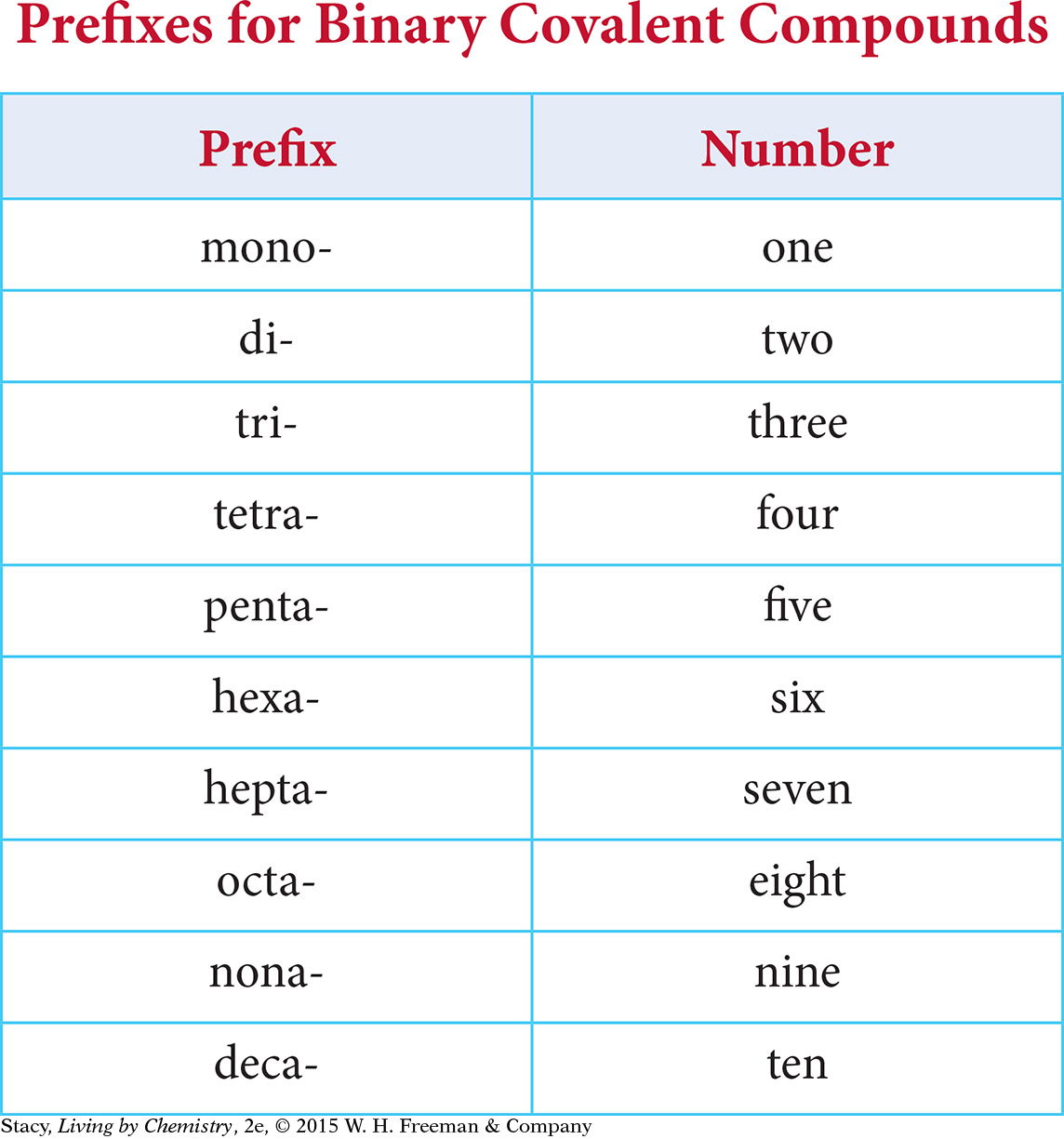
If you know the formula for a binary covalent compound, some simple rules can help you to name it correctly.
Write the name of the first element in the formula. Use a prefix in front of the element name to indicate if there are two or more atoms of that element in the formula.
Write the name of the second nonmetal in the formula and change the ending of the name to the suffix -ide. Use a prefix to indicate how many atoms of that element are in the formula, even if there is only one.
The table lists common prefixes and the number of atoms the prefix represents in the formula for a binary covalent compound.
The first molecular covalent compound in the binary compounds table on page B-1 is CO2. The compound is named carbon dioxide following the rules for naming binary covalent compounds. Notice that this name helps to distinguish CO2 from carbon monoxide, CO, which has one oxygen atom in the formula. Notice also that the prefix mono- is used in carbon monoxide to indicate that there is only one oxygen atom in that compound but that the prefix is not used in front of carbon in the name of either compound.
FORMULAS OF BINARY COVALENT COMPOUNDS
If you know the name of a binary molecular compound, you can determine the correct chemical formula.
Write the symbol for the first element in the name. Use a subscript to indicate the number of atoms of that element in the formula, based on the prefix in the name.
Write the symbol for the second element. Use a subscript to indicate the number of atoms of the second element, based on the prefix in the name.
Remember that, in formulas, the numeral 1 is not written as a subscript.
For example, the compound dinitrogen tetroxide contains nitrogen, N, and oxygen, O. The di- prefix indicates that there are two nitrogen atoms in the formula and the tetra- prefix indicates that there are four oxygen atoms in the formula. (Note that in this case, the “a” is dropped.) So the correct formula is N2O4.
Practice Exercises
Write the name for these binary covalent compounds.
CO2
S2O
PCl3
N2O
SO2
NF3
Write the formula for these binary covalent compounds.
dinitrogen tetrafluoride
dinitrogen pentoxide
carbon monoxide
diphosphorus trisulfide
carbon tetrabromide
sulfur trioxide
Answers
1a. carbon dioxide
1b. disulfur monoxide
1c. phosphorus trichloride
1d. dinitrogen monoxide
1e. sulfur dioxide
1f. nitrogen trifluoride
2a. N2F4
2b. N2O5
2c. CO
2d. P2S3
2e. CBr4
2f. SO3
NAMES OF BINARY IONIC COMPOUNDS
If you know the formula for a binary ionic compound, some simple rules can help you to name it correctly. Recall that a binary ionic compound is a combination of a positively charged metal cation and a negatively charged nonmetal anion.
Write the element name of the cation followed by the element name of the anion.
Change the ending of the element name of the anion to the suffix -ide.
For example, NaCl is the formula for sodium chloride, and CaCl2 is the formula for calcium chloride. Notice that no prefixes are used in naming binary ionic compounds.
FORMULAS OF BINARY IONIC COMPOUNDS
If you know the name of a binary ionic compound, you can use it to determine the correct chemical formula.
Write the symbol for the cation, including the charge.
Write the symbol for the anion, including the charge.
Use subscripts in the formula so the net charge of the compound is zero.
Remember not to use a subscript to indicate the numeral 1, and do not include charges in the formula.
For example, the compound strontium chloride is made up of a combination of the cation strontium, Sr2+, and the anion chloride, Cl–. For the net charge to be zero, the formula for strontium chloride requires one strontium cation and two chloride anions:
1(1+2) + 2(–1) = (+2) + (–2)=0
So the formula for strontium chloride is SrCl2.
Practice Exercises
Write the name for the following binary ionic compounds.
KBr
MgO
Na3P
NaCl
Ca3N2
LiS2
Write the formula for the following binary ionic compounds.
calcium sulfide
beryllium nitride
aluminum oxide
potassium oxide
strontium sulfide
barium bromide
Answers
1a. potassium bromide
1b. magnesium oxide
1c. sodium phosphide
1d. sodium chloride
1e. calcium nitride
1f. lithium sulfide
2a. CaS
2b. Be3N
2c. Al2O
2d. K2O
2e. SrS
2f. BaBr2
NAMES OF IONIC COMPOUNDS WITH TRANSITION METALS
Unlike main group atoms, most transition metals can have more than one ion charge. When naming ionic compounds that contain transition metals, Roman numerals are used to indicate the charge on the cation. If you know the formula for an ionic compound that contains a transition metal, you can determine its name.
Follow the same basic rules for naming binary ionic compounds. Name the cation first, followed by the anion, changing the ending of the element name of the cation to the suffix -ide.
To indicate the charge on the cation, use a Roman numeral in parentheses. Use the rule of zero charge and the charge on the anion to determine the charge on the cation.
Note that if the compound contains silver, Ag, or zinc, Zn, there is no need to use a Roman numeral in the name. Silver typically has a charge of +1 and zinc has only one cation with a charge of +2.
For example, the formula for Fe2O3 contains three oxide anions, each with a charge of −2. The formula shows that there are two Fe ions. For the net charge on the compound to be zero, each iron cation must have a charge of +3:
2(+3) + 3(–2) = (+6) + (–6) = 0
So, the name of this compound is iron (III) oxide.
FORMULAS OF IONIC COMPOUNDS WITH TRANSITION METALS
If you know the name of an ionic compound that contains a transition metal, you can use the name to determine the correct formula.
Write the symbol for the metal cation. The Roman numeral in parentheses indicates the charge on the cation. Remember that silver has a charge of +1 and zinc has a charge of +2.
Write the symbol for the anion, including the charge.
Use subscripts in the formula to indicate that the net charge of the compound is zero.
Page B-5Remember not to use a subscript to indicate the numeral 1, and do not include charges in the formula.
For example, the compound chromium (III) oxide contains a chromium cation, Cr3+ as indicated by the Roman numeral III in parentheses. The oxide anion has a charge of (−2). Using the rule of zero charge, you can determine how many of each ion is in one formula unit of chromium oxide.
2(+3) + 3(–2) =(+6) + (–6) = 0
So the formula contains two chromium cations and three oxygen anions: Cr2O3.
Practice Exercises
Write the name for the following binary ionic compounds.
CuF2
AgCl
FeCl3
PbS
SnO2
Mn3P2
Write the formula for the following binary ionic compounds.
cobalt (III) chloride
chromium (IV) sulfide
copper (I) nitride
zinc oxide
cobalt (II) iodide
lead (IV) sulfide
Answers
1a. copper (II) fluoride
1b. silver chloride
1c. iron (III) chloride
1d. lead (II) sulfide
1e. tin (IV) oxide
1f. manganese (II) phosphide
2a. CoCl3
2b. CrS2
2c. Cu3N
2d. ZnO
2e. CoI2
2f. PbS2
NAMES OF IONIC COMPOUNDS WITH POLYATOMIC IONS
Some ionic compounds contain ions made up of two or more elements called polyatomic ions.
Follow the basic rules for naming binary ionic compounds.
Insert the name of any polyatomic ions in the formula. There is a list of common polyatomic ions in the reference tables at the end of your textbook.
For example, Ca(NO3)2 contains more than two elements, so it’s not a binary ionic compound. It’s a compound that contains the polyatomic ion nitrate, (NO3)–. The metal cation is calcium, so the name of this compound is calcium nitrate.
FORMULAS OF IONIC COMPOUNDS WITH POLYATOMIC IONS
If you know the name of an ionic compound containing a polyatomic ion, you can use it to determine the correct chemical formula. Remember that, in the formula, the charge on the polyatomic ion is for the entire group of atoms as a unit. When there is more than one of the same polyatomic ion in a formula, the ion is enclosed in parentheses and a subscript number indicates how many ions are in the compound. Follow the same rules for writing formulas for other types of compounds, but make sure polyatomic ions are treated as a unit.
Write the symbol for the cation, including the charge.
Write the symbol for the anion, including the charge.
Use subscripts in the formula so the net charge of the compound is zero.
For example, in the compound magnesium hydroxide, the cation is magnesium, Mg2+, and the anion is hydroxide, (OH)–. For the charge on the compound to be zero, the formula must contain one magnesium ion and two hydroxide polyatomic ions:
1(+2) + 2 (–1) = (+2) + (–2) = 0
So the formula for magnesium hydroxide is Mg(OH)2. Notice that parentheses and a subscript are needed to show that there are two hydroxide units in the formula. An ionic compound could contain both a transition metal ion and a polyatomic ion. If that’s the case, follow the rules for both transition metal ions and polyatomic ions.
Practice Exercises
- Write the name for the following ionic compounds with polyatomic ions.
Ca(NO3)2
Ag2SO4
Ba(OH)2
CuCO3
Ca3(PO4)2
Pb(HCO3)4
- Write the formula for the following ionic compounds with polyatomic ions.
calcium bromate
ammonium hydroxide
potassium nitrate
nickel (II) cyanide
iron (III) sulfate
copper (II) phosphate
Answers
1a. calcium nitrate
1b. silver sulfate
1c. barium hydroxide
1d. copper (II) carbonate
1e. calcium phosphate
1f. lead (IV) hydrogen carbonate
2a. Ca(BrO3)2
2b. NH4OH
2c. KNO3
2d. Ni(CN)2
2e. Fe2(SO4)3
2f. Cu3(PO4)2
NAMES OF ACIDS
In Unit 4: Toxins, you learned that acids could be defined as molecules that break apart, or dissociate, in solution to form at least one hydrogen cation, H+, and an anion. Examine the table of acids. Try to find patterns in the names, formulas, and ions in solution.
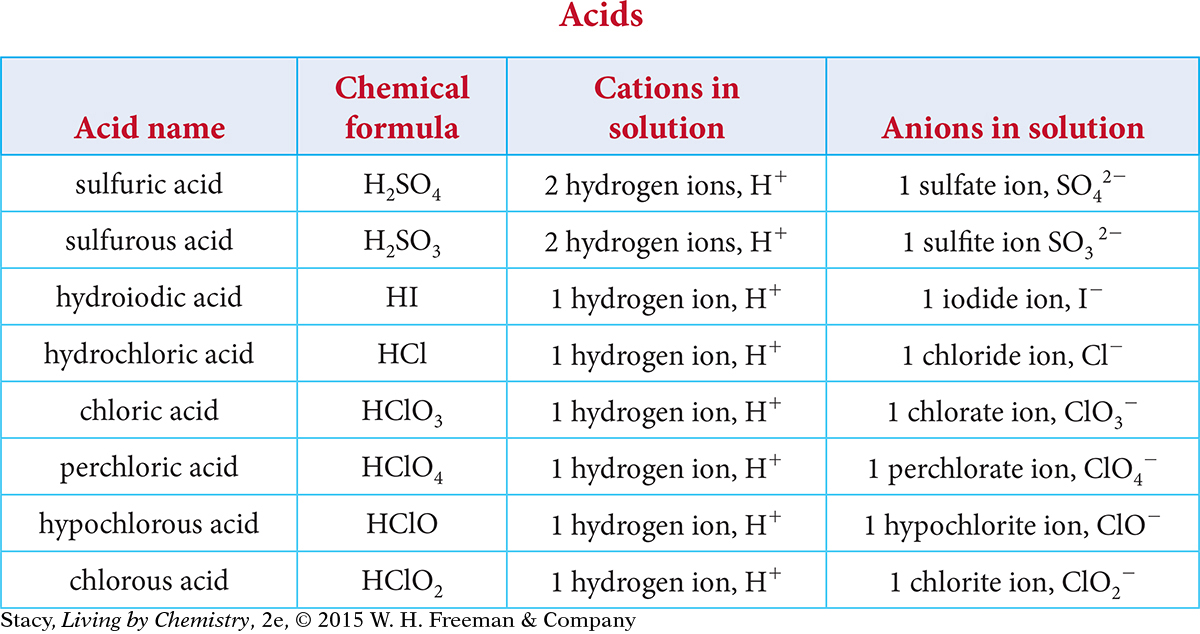
Here are some patterns you might notice.
The formula for each acid contains the element hydrogen, and each acid dissociates to form H+ ions in solution.
When dissociated in solution, some of the anions are polyatomic ions, while others are monatomic ions.
The names of acids containing only two elements begin with “hydro.”
The names of the acids with polyatomic ions start with the root of the name of the polyatomic ion.
Some acids have a name that ends -ic acid; others have names that end in -ous acid.
There is no net charge on the compound before it dissociates, and the net charge of the ions in solution is zero.
NAMES OF BINARY ACIDS
In the table, some of the acids are made up of covalent molecules that contain only two elements. These compounds dissociate into hydrogen ions and nonmetal anions that are not polyatomic ions. To name these binary acids, you need to know the root of the name of the anion. For example, the root of the chloride ion is chlor-, and the root of the iodide ion is iod-.
Add the prefix hydro- to the root name of the anion.
Add the suffix -ic to the root of the anion name.
Add the word acid to the name.
For example, the molecule HBr is an acid that dissociates into H+ and Br– ions in solution. The root of the anion name is brom-, so the name of this acid is hydrobromic acid. Likewise, HF is hydrofluoric acid.
FORMULAS OF BINARY ACIDS
Acids have formulas that begin with hydrogen, H. Binary acids are named using the root of the anion that forms in solution, so you can work backward from the root to determine the name.
Identify the root of the anion name by removing the prefix hydro- and the suffix -ic from the root of the anion name. Rename the anion using the suffix -ide.
Write the symbol for the anion, including the charge.
Write the symbol for the hydrogen ion, including the charge.
Use subscripts to write a chemical formula for a molecule that has no charge and when dissociated in solution has no net charge.
For example, if you remove the prefix hydro- and the suffix -ic from hydrobromic acid, you are left with the root, brom-. Adding back the -ide ending indicates that the anion is bromide, Br–. This acid is a molecule with no charge that dissociates into hydrogen ions, H+, and Br– anions so the formula can be determined using the charges of the ions in solution, such that they add to zero charge.
1(+1) + 1(–1) = 0
This means that when one molecule of this acid dissociates, one hydrogen ion and one bromide ion form in solution, so the compound is the molecule, HBr. Consider hydroselenic acid. The root of the anion name is selen-, which corresponds to the selenide ion, Se2–. For the solution to have no net charge, the molecule must dissociate into 2 hydrogen ions, H+ and 1 selenide ion, Se2–.
2(+1) + 1 (–2) = (+2) + (–2) = 0
So the formula for this acid is H2Se.
NAMES OF ACIDS THAT CONTAIN POLYATOMIC ANIONS WITH OXYGEN
In the table, some of the acids are made up of covalent molecules that dissociate into hydrogen ions and polyatomic anions that contain oxygen. To name these acids, you use the root of the name of the polyatomic anion. For example, the root of the sulfate ion is sulf- and the root of the chlorite ion is chlor-.
Identify the root of the name of the polyatomic anion.
If the polyatomic anion ends in -ate, add the suffix -ic to the root of the polyatomic anion name.
If the polyatomic anion ends in -ite, add the suffix -ous to the root of the polyatomic anion name.
Add the word acid to the name.
For example, HClO dissociates into one hydrogen ion, H+, and the polyatomic ion hypochlorite, ClO–, which has the root, hypochlor-. Since the polyatomic anion ends in -ite, the suffix -ous is added to the root, and the word acid is added to form hypochlorous acid. As another example, HClO2 dissociates into one hydrogen H+ ion and the polyatomic ion chlorite, with the root, chlor-. Because the anion name ends in -ite, this acid is called chlorous acid. Likewise, HClO4 with the perchlorate ion is perchloric acid, and HClO3 with the chlorate ion is chloric acid.
FORMULAS OF ACIDS THAT CONTAIN POLYATOMIC ANIONS WITH OXYGEN
Remember that these acids are molecules with no charge that dissociate into hydrogen ions and polyatomic anions that contain oxygen in solution. They are named using the root of the polyatomic anion that forms in solution. So work backward from the root of the anion to determine the name.
Identify the name and charge of the polyatomic ion from the root of the anion name.
Write the symbol for the polyatomic anion, including the charge.
Write the symbol for the hydrogen ion, including the charge.
Use subscripts to write a chemical formula for a molecule that has no charge and when dissociated in solution has no net charge.
For example, sulfuric acid does not have the prefix hydro-, so it must contain a polyatomic ion. Both sulfite, (SO3)2– and sulfate (SO4)2– have the root sulf-, but the name of this acid ends in -ic. The correct anion is sulfate, because its name ends in -ate. The sulfate ion has a charge of +2, so when it dissociates, the original molecule must break apart into two hydrogen ions, H+, and one sulfate ion, (SO4)2–.
2(+1) +1(–2) = (1+2) + (–2) = 0
So the formula for this acid contains two hydrogen atoms and one sulfate ion, H2SO4.
Practice Exercises
- Write the name for these acids.
HBr
H2SO4
HNO3
HCl
HBrO
H3PO3
- Write the formula for these acids.
carbonic acid
hydrofluoric acid
phosphoric acid
sulfurous acid
perbromic acid
nitrous acid
Answers
1a. hydrobromic acid
1b. sulfuric acid
1c. nitric acid
1d. hydrochloric acid
1e. hypobromous acid
1f. phosphorous acid
2a. H2CO3
2b. HF
2c. H3PO4
2d. H2SO3
2e. HBrO4
2f. HNO2
NAMES AND FORMULAS FOR ARRHENIUS BASES
According to the Arrhenius theory of acids and bases, a base is defined as a substance that adds OH– to an aqueous solution. Examine the table of Arrhenius bases.
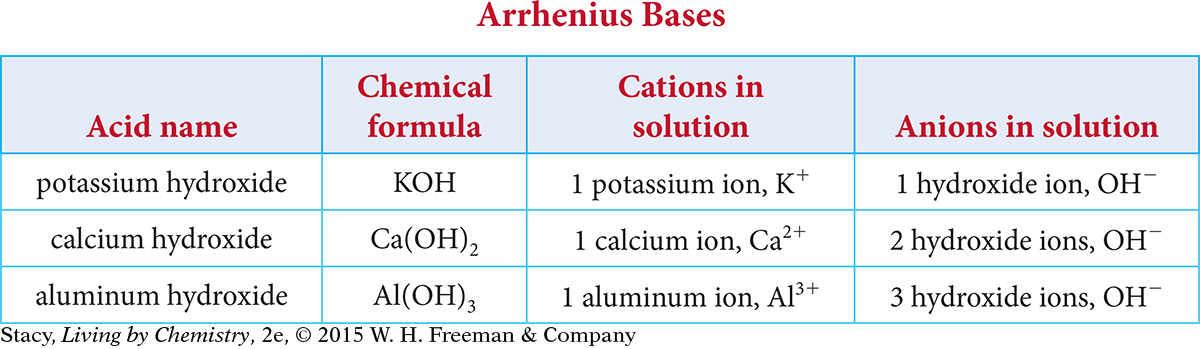
Here are some patterns you might notice.
They each contain the hydroxide polyatomic ion.
They each dissociate into metal cations and hydroxide polyatomic anions.
There are as many hydroxide ions as the charge on the metal.
The names all contain the word hydroxide.
When naming and writing formulas for Arrhenius bases, you simply follow the rules for naming ionic compounds that contain a polyatomic ion. For these bases, the polyatomic ion is the hydroxide ion, OH–.
Practice Exercises
Write the name for these bases.
Mg(OH)2
NaOH
Sr(OH)2
Write the formula for these bases.
potassium hydroxide
barium hydroxide
lithium hydroxide
Answers
1a. magnesium hydroxide
1b. sodium hydroxide
1c. strontium hydroxide
2a. KOH
2b.Ba(OH)2
2c. LiOH