Percent Composition
Elements combine in specific ratios to form compounds, so the ratio of one element to another in a compound is always the same. For example, water, H2O, is made up of the elements hydrogen and oxygen in a 2:1 ratio. If the ratio of hydrogen to oxygen were different, the compound would not be water. For example, hydrogen peroxide, H2O2, is also made up of hydrogen and oxygen atoms, but the ratio of hydrogen to oxygen in this compound is 1:1. In one mole of water, there are always two moles of hydrogen atoms and one mole of oxygen atoms, as indicated by the ratios of elements in the chemical formula.
Examine the table that shows the composition of water in terms of moles, mass, and percent mass of each element in the compound.
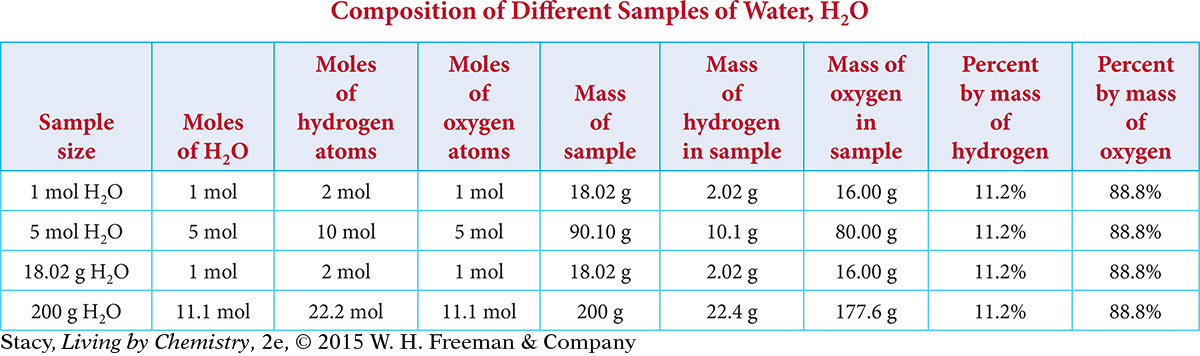
Notice that for each sample size of water, the percent of hydrogen in the sample and the percent of oxygen in the sample are always the same. This is because these elements always combine in the same molar ratio to form water. The percent composition of elements in a compound is the percent by mass of each element in the compound. A percent is an amount per 100. You can calculate a percent by taking part of a whole amount, dividing it by the whole amount, and multiplying by 100%.
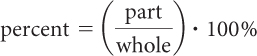
In terms of percent composition of elements in a compound, the “part” is the mass of one of the elements in a sample of a compound and the “whole” is the mass of the entire sample of the compound. If you add the masses of each element present in the sample of the compound, the result is the mass of the compound. If you add the percent by mass of each element in a compound the total should be 100%.
DETERMINING PERCENT COMPOSITION USING THE CHEMICAL FORMULA
If you know the chemical formula for a compound, you can determine the percent composition of each element in it by using the mass of one mole of the compound and the mass of each element that would be found in one mole of the compound.
Example 1
Percent Composition of CO
What is the percent composition of carbon monoxide, CO?
Solution
Assume that you have one mole of CO.
Step 1: Use the periodic table to calculate the molar mass of CO.
molar mass of CO = 12.01 g/mol + 16.00 g/mol = 28.01 g/mol
One mole of CO is 28.01 g.
Step 2: Determine the percent mass of each element in the compound.
Divide the mass of each element in one mole of the compound by the mass of one mole of the compound and multiply by 100%. The molar ratio of C:O in the compound is 1:1, so there is one mole of each element in one mole of CO.
The mass of one mole of carbon, C, is 12.01 g. The mass of one mole of oxygen, O, is 16.00 g. Use these values to determine the percent mass of each compound in one mole of the compound.
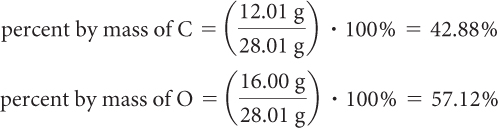
Step 3: Check your answer by adding the percent by mass of each element. The total should be 100%.
42.88% + 57.12% = 100.00% (or 100% when rounded to the nearest whole number)
Example 2
Percent Mass of Oxygen in KClO3
What is the mass of oxygen that can be produced from the decomposition of 150.0 g of potassium chlorate, KClO3? (Hint: Find the percent composition of oxygen in the compound to figure out the answer.)
Solution
When potassium chlorate, KClO3, decomposes, the oxygen produced will be the amount present in the 150.0 g sample of the compound.
Step 1:Calculate the mass of one mole of potassium chlorate, KClO3.
molar mass of KClO3 = 1(39.10 g/mol) + 1(35.45 g/mol) +3(16.00 g/mol)
= 39.10 g/mol + 35.45 g/mol + 48.00 g/mol
= 122.55 g/mol
One mole of potassium chlorate, KClO3, is 122.55 g.
Step 2: Determine the percent mass of oxygen, O, in one mole of the compound.
The mass of one mole of oxygen is 16.00 g. In one mole of potassium chlorate, KClO3, there are three moles of oxygen, O.
mass of O in one mole of compound = 3(16.00 g)
= 48.00 g
One mole of potassium chlorate, KClO3,is = 122.55 g
Divide the mass of oxygen, O, in one mole of potassium chlorate, KClO3, by the molar mass of the potassium chlorate, KClO3, and multiply by 100%.
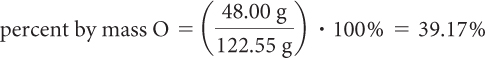
Step 3: Use the percent composition of oxygen, O, to determine the amount of oxygen produced in the decomposition reaction.
The mass of oxygen produced when 150.0 g of KClO3 decomposes is 39.17% of 150.0 g. Convert the percentage to a decimal and multiply by 150.0 g.
0.3917 × 150.0g = 58.75 g
The decomposition of 150.0 g of potassium chlorate, KClO3, produces 58.75 g of oxygen, O
Practice Exercises
What is the percent composition of HCl?
What is the percent composition of ammonium phosphate (NH4)3PO4?
What is the mass of aluminum needed to produce 298 g of Al2(SO4)3?
Answers
H = 2.7%, Cl = 97.3%
2.N = 28.19%, H = 8.113%, P = 20.77%, O = 42.93%. The percent by mass values add to 100.10% or 100% if rounded to the nearest whole number.
47.0 g Al