Molecular and Empirical Formulas
In Unit 2: Smells, you learned that the chemical formula for a molecular covalent compound is also called the molecular formula. The molecular formula indicates the number of atoms of each element present in one molecule of a compound. Examine the table of formulas. Notice that for each compound, there is a molecular formula and what is called the empirical formula.
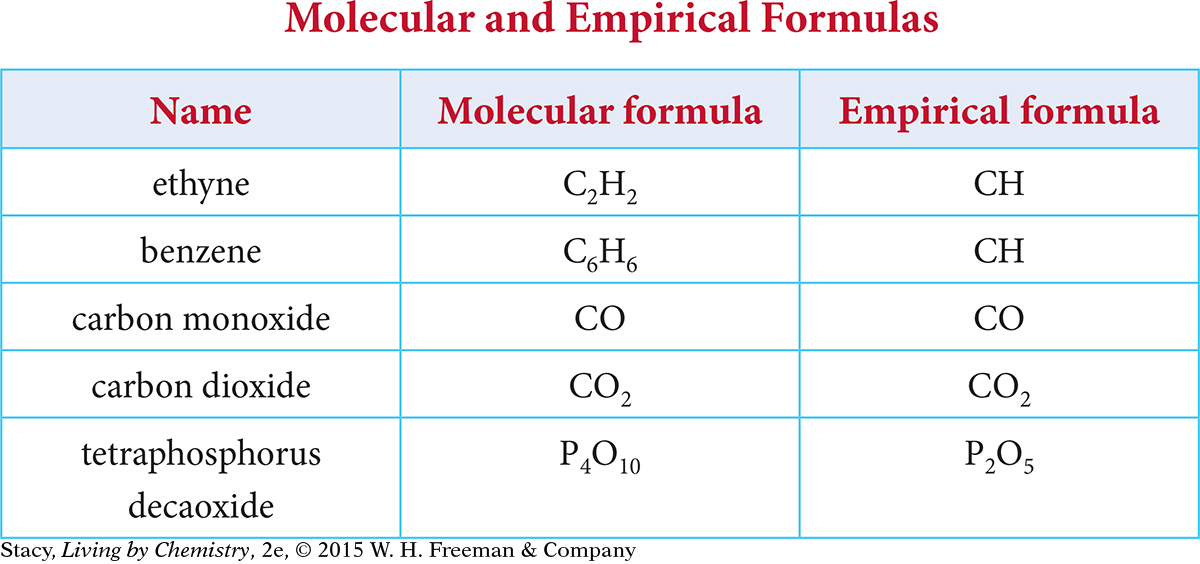
Here are some patterns you might notice.
For each compound, the molecular formula and the empirical formula both have the same type of atoms.
Some compounds have a molecular formula that is different from the empirical formula.
Some compounds have a molecular formula that is the same as the empirical formula.
The molecular formula and the empirical formula are related mathematically.
If the empirical formula is different from the molecular formula, you can multiply the subscripts in the empirical formula by a single number to get the values for subscripts in the molecular formula. For example, if you multiply the subscripts for CH by 6, you get the molecular formula for benzene, C6H6. You can multiply the subscripts in P2O5 by 2 to get the molecular formula P4O10.
The empirical formula is the simplest formula for a compound and shows the smallest whole-number ratio of the atoms present in that compound. There is a relationship between molecular formula and empirical formula that can be seen in the patterns of the subscripts. If you multiply the subscripts in the empirical formula by the number of empirical units, you get the molecular formula. Remember if there is no subscript, the value is 1.
(empirical formula)(number of empirical units) = molecular formula
| ethyne: | (CH)2 = C2H2 |
| benzene: | (CH)6 = C6H6 |
| carbon monoxide: | (CO)1 = CO |
| carbon dioxide: | (CO2)1 = CO2 |
If the molecular formula is different from the empirical formula, you can determine the empirical formula by dividing each subscript by the largest whole-number factor they have in common. For example, you can divide subscripts 4 and 10 in P4O10 by the number 2 to get the empirical formula P2O5.
Example 1
Determining Empirical Formula from Molecular Formula
Write the empirical formula for these compounds.
C6H12O2
C4H8O2
H2SO4
Solution
The largest whole-number factor the subscripts have in common is 2. You can divide each subscript by 2 to determine that the empirical formula is C3H6O.
The largest whole-number factor the subscripts have in common is 2. You can divide each subscript by 2 to determine that the empirical formula is C2H4O.
The largest whole-number factor the subscripts have in common is 1. You can divide each subscript by 1 to determine that the empirical formula is H2SO4, which is the same as the molecular formula.
If you know the amount in grams of each element present in a sample of a compound, or you know the percent composition for a compound, you can determine its empirical formula. If you also know the molar mass of the compound, you can use it along with the empirical formula to determine the molecular formula of the compound.
Example 2
Determining the Molecular Formula Using Mass Data
A 500.0 g sample of hexanoic acid contains 310.2 g of carbon, 52.1 g of hydrogen, and 137.8 g of oxygen. The molar mass of this compound is 116.16 g/mol. Determine the empirical and molecular formulas for this compound.
Solution
Step 1: The empirical and molecular formulas show the relative numbers of atoms in an element, not the relative amount of mass in each element. So the first step is to convert the amount of each element in grams to the amount in moles. You can do this using the molar mass of the element from the periodic table.
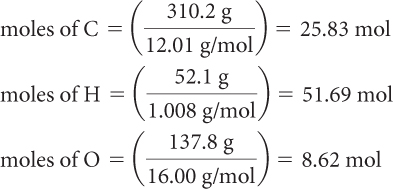
Step 2: The molecular formulas show the relative amounts of moles in whole-number ratios. To convert the amounts of moles to whole numbers, divide each value by the lowest number of moles.
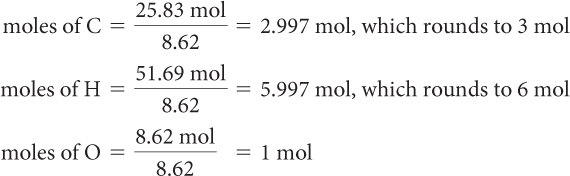
Use the ratio of moles, 3:6:1, to write the empirical formula, C3H6O.
Step 3: You can calculate the number of empirical units by dividing the molar mass of the compound by the empirical molar mass. The empirical molar mass is the sum of the molar masses of the elements in the empirical formula.
empirical molar mass = 3 (12.01 g/mol) + 6 (1.008 g/mol) + (16.00 g/mol)
= 58.08 g/mol
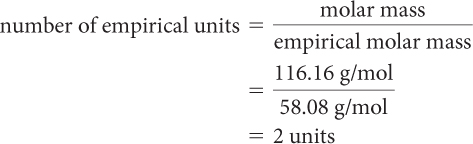
(empirical formula)(number of empirical units) = molecular formula
(C3H6O)2 = C6H12O2
The molecular formula for the compound is C6H12O2.
Example 3
Determining the Molecular Formula Using Percent Composition Data
A compound contains 71.65% Cl, 24.27% C, and 4.07% H. The molar mass of the compound is 98.96 g/mol. Determine the empirical formula and molecular formula for this compound.
Solution
Step 1: When amounts in mass are not provided, assume that you have 100 g of the compound. Find the mass of each element in grams by converting the percent values to decimals and multiplying by the total mass.
mass of Cl = 0.7165 · (100 g) = 71.65 g of Cl
mass of C = 0.2427 · (100 g) = 24.27 g of C
mass of H = 0.0407 · (100 g) = 4.07 g of H
Step 2: Convert the amounts in grams to moles.
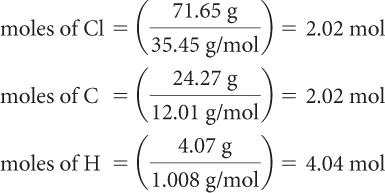
Step 3: Convert the amounts in moles to whole numbers by dividing by the lowest value.
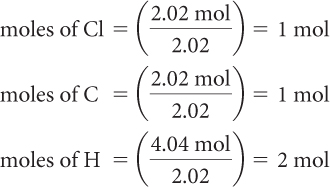
The empirical formula is CH2Cl.
Step 4: Determine the empirical molar mass.
empirical molar mass = (12.01 g/mol) + 2(1.008 g/mol) + (35.45 g/mol)
= 49.48 g/mol
Step 5: Calculate the number of empirical units.
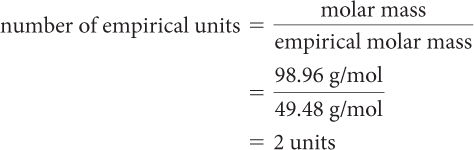
Step 6: Multiply the subscripts in the empirical formula by the number of empirical units to determine the molecular formula.
empirical formula)(number of empirical units) = molecular formula
(CH2Cl)2 = C2H4Cl2
The molecular formula for the compound is C2H4Cl2.
Practice Exercises
What is the molecular formula for a compound with an empirical formula of C4H9 and a molar mass of 114.22 g/mol?
A compound has a mass percent composition of 5.927% hydrogen and 94.073% oxygen. The molecular mass of this compound is 34.016 g/mol. Find both the empirical and molecular formulas.
A compound has a mass percent composition of 40.00% carbon, 6.72% hydrogen, and 53.28% oxygen. The molecular mass of this compound is 150.13 g/mol. Determine both the empirical and molecular formulas.
Page B-17A compound contains 0.384 g carbon, 0.048 g hydrogen, and 0.5674 g chlorine. The molecular mass of this compound is 125 g/mol. Calculate the empirical and molecular formulas.
Answers
C8H18
empirical formula: HO, molecular formula: H2O2
empirical formula: CH2O, molecular formula: C5H10O5
empirical formula: C2H3Cl, molecular formula: C4H6Cl2