Unit 3: Weather
Lesson 49
Possible answer: To have weather, a planet must have a layer of gases surrounding its surface.
A physical change is a change in the form of a substance that does not change the identity of the substance.
Lesson 50
Possible answer: One way to determine the volume of 3 cm of water in the rain gauge is to use the graph. Find 3 cm on the x-axis, move up to the line on the graph, and then read the corresponding y-value. A second way is to use a proportionality constant. Examining the data in the table shows that the value of the volume is always twice the value of the height.
Possible answer: Both the volume and height of water in the rain gauge will keep track of the increase because they both increase by the same proportion as additional rain falls into the gauge.
Possible answer: The three containers would have different volumes of water in them after the storm because their bases have different areas. The greatest volume of water will be in the washtub and the least will be in the graduated cylinder. However, the height of water in each container will be the same.
Lesson 51
You can convert the volume of snow in a snowpack to a volume of water by using the mathematical equation for density,
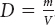 . If you know the volume and density of snow, you can determine the mass of the snow in the snowpack. If the snow melts, the mass of the water will be the same as the mass of the snow. Then you can calculate the volume of water by dividing the mass by the density of water, which is equal to 1 g/mL.
. If you know the volume and density of snow, you can determine the mass of the snow in the snowpack. If the snow melts, the mass of the water will be the same as the mass of the snow. Then you can calculate the volume of water by dividing the mass by the density of water, which is equal to 1 g/mL.
16.1 g
17.5 g
4.3 g of iron occupies a greater volume because volume increases as density decreases, and iron is less dense than lead.
2.6 mL of lead has a larger mass because mass increases as density increases, and lead is more dense than iron.
Lesson 52
Possible answer: Liquids usually expand when they are heated and contract when they are cooled. At any given temperature the volume of a fixed amount of liquid will always be the same. If the liquid is in a tube, the height of the column of liquid will change in a predictable way with changes in temperature.
–40 ºF
Normal body temperature is about 98.6 ºF. A body temperature of 40 ºC is the same as 104 ºF, which indicates fever and probable illness.
50 ºF
13 ºC
Lesson 53
Possible answer: Absolute zero is the temperature at which the volume of a gas would be equal to zero. It is considered a theoretical temperature because a real gas would condense into a liquid and then a solid before reaching absolute zero, making a volume of zero impossible.
Possible answer: The kinetic theory of gases defines the temperature of a gas as the average kinetic energy of the particles of the gas.
The smallest unit is 1 ºF because there are 180 Fahrenheit degrees between the freezing temperature of water and the boiling temperature of water, while there are only 100 Celsius degrees or kelvins between the freezing temperature of water and the boiling temperature of water.
–173 ºC
333 K
–23 ºC
298 K
27 ºC
173 K
127 ºC
308 K
450 K
258 K
B
Lesson 54
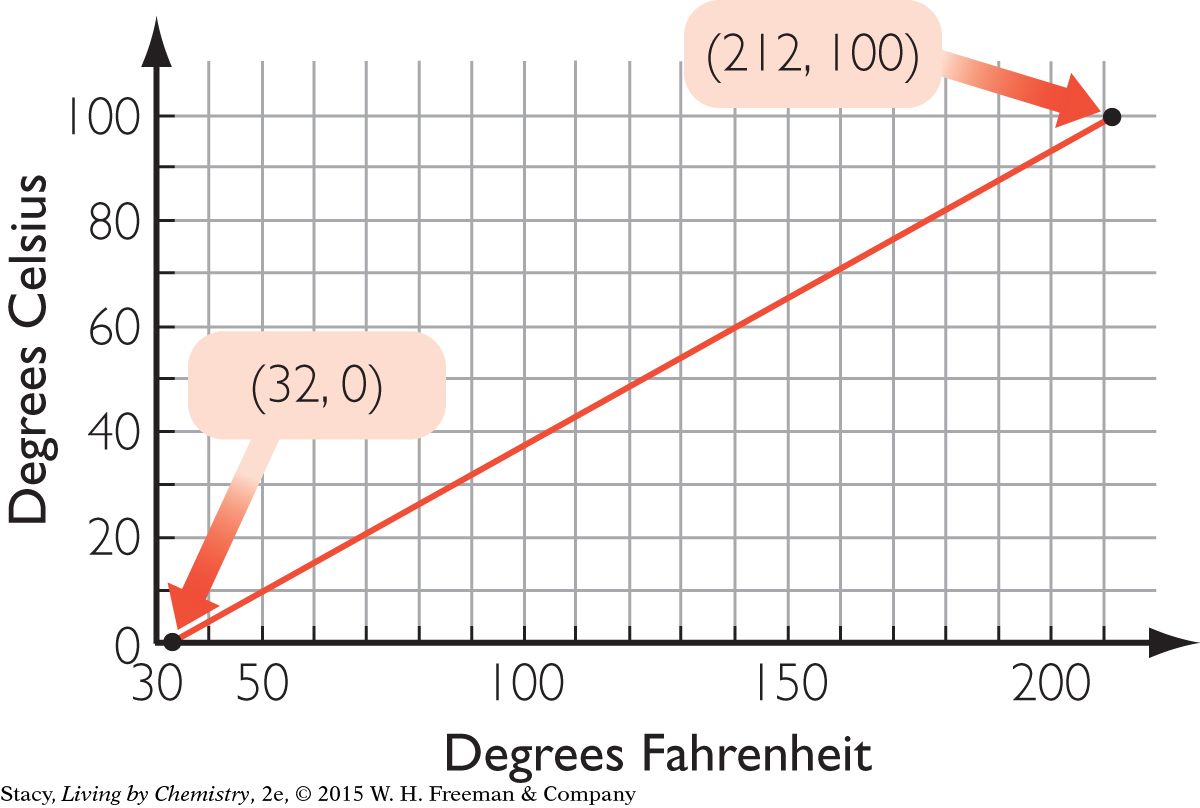
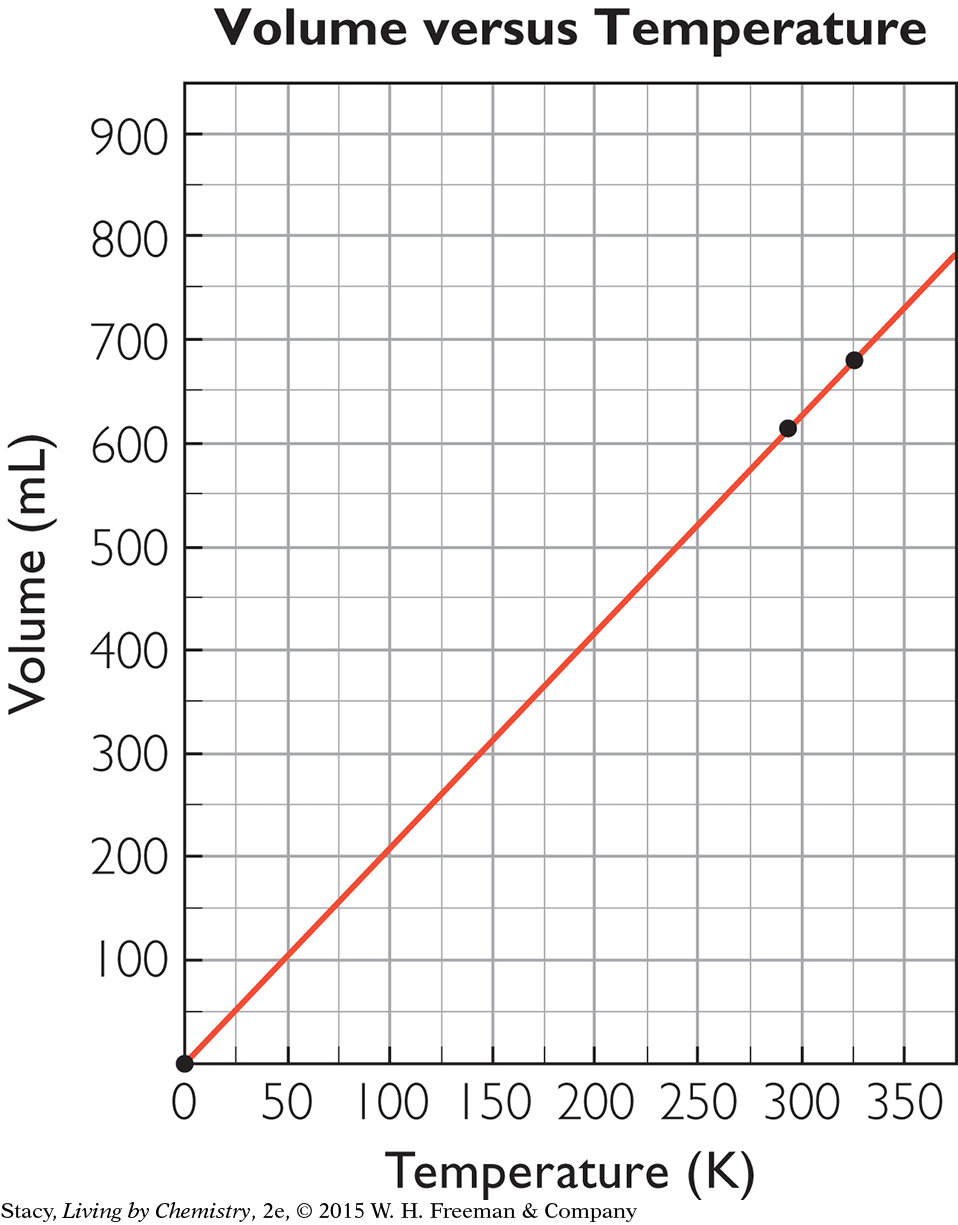
Determine the proportionality constant, k, for a gas by dividing the volume of the gas by its temperature measured on the Kelvin temperature scale.
689 mL
The answer is reasonable because 325 K corresponds to about 700 mL.
186 K
Lesson 55
When air gets warmer, its volume increases and its density decreases. Air that is less dense than the surrounding air will rise.
A
Chapter 10 Review Exercises
283 K
760 mL
3.3 mL
2700 mL. The volume of gas can be calculated using the density equation. The mass of the carbon dioxide does not change when it changes phase and becomes a gas.
Lesson 56
The density of a gas is much lower than the density of a solid.
Possible answer: Sublimation occurs when a substance changes from the solid phase directly to the gas phase without becoming a liquid. Evaporation occurs when a substance changes from the liquid phase to the gas phase.
Possible answer: Ice floats in water so it must have a slightly lower density than water.
4.1 mL
12 g
Lesson 57
Because the air inside the balloon exerts pressure on the inside wall but there is no pressure balancing it on the outside wall, the balloon will expand and most likely pop.
Lesson 58
As you push down on the plunger of the syringe, the gas pressure inside the syringe increases and the pressure on the scale increases. The pressure inside the syringe is equal to the pressure you are exerting on the plunger, which can be read from the scale. To calculate the pressure of the gas, divide the weight on the scale by the surface area of the plunger. Then add atmospheric pressure.
Possible answer: The relationship between gas pressure and gas volume is different from the relationship between gas volume and gas temperature because an increase in pressure causes the volume to decrease, while an increase in temperature causes the volume to increase.
Lesson 59
Possible answer: You can change the pressure, the volume, or the temperature of a gas sample.
720 L
The volume of air inside the bottle stays the same because the bottle is a rigid container.
Yes, the air inside the bottle will lose energy until it has the same temperature as its surroundings because glass does not insulate against temperature changes.
Pressure and temperature are directly proportional. The pressure of the air inside the bottle will decrease as its temperature decreases.
0.93 atm
Lesson 60
According to the kinetic theory of gases, increasing the gas volume decreases the gas pressure because the molecules of gas have more room in which to travel and therefore they strike the walls of the container less often.
Possible answer: An increase in temperature probably caused the change in pressure because pressure and temperature are proportional and the volume of a gas cylinder doesn’t change.
The volume of a gas cannot decrease to zero because the particles of the gas themselves occupy space.
The volume of your lungs at 3.5 atm is much lower than the volume at sea level.
Holding your breath when you ascend quickly is dangerous because the pressure decreases rapidly as you rise. If you hold your breath, your lungs will act as sealed containers, making the pressure inside the lungs much greater than the pressure outside the lungs, possibly causing your lungs to rupture.
Lesson 61
The combined gas law is a mathematical equation that relates gas pressure, temperature, and volume. This law applies when all three variables can change at the same time.
The volume of the gas will decrease because pressure and volume are inversely proportional when the temperature and the amount of gas remain unchanged.
PV =k
0.49 L
The volume of the balloon will increase because, based on the values given, the change in pressure has a greater effect on the final volume than the change in temperature.
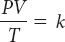
1.6 L
Lesson 62
Possible answer: High-pressure areas are generally associated with clear skies. Low-pressure areas are generally associated with cloudy weather and precipitation.
Chapter 11 Review Exercises
The pressure of a gas is proportional to the temperature of the gas. As a gas becomes warmer, its molecules move faster and collide with each other and other objects more frequently.
0.80 atm
2.0 atm
Lesson 63
Possible answer: Air pressure decreases with increasing altitude because the number of molecules of air in a given volume decreases. This means that there are fewer collisions of air molecules with one another and with other objects, and therefore the air exerts less pressure.
Possible answers: Increase the amount of air in the tire. Increase the temperature of the air in the tire. Press on the outside of the tire to decrease its volume.
When a high-pressure system moves into a region, the air pressure increases. When the air pressure on the open end of the barometer increases, it pushes on the mercury harder than it did before. Mercury is pushed higher into the closed end of the barometer until the downward pressure caused by the weight of the column of mercury is equal to the atmospheric pressure.
Lesson 64
Possible answer: Chemists invented the mole so as to have a sufficiently large unit with which to count the enormous number of particles in a sample of gas.
3.Each gas sample contains 1 mol of atoms.
0.0446 mol/L
The xenon sample has the largest mass because the mass of each xenon atom is greater than the mass of each neon atom or argon atom.
the xenon sample
8.0 g of helium
The two balloons hold the same volume. The volumes of the balloons are the same because the number of moles of He is equal to the number of moles of O2.
Lesson 65
The ideal gas law is an equation that relates the variables pressure, volume, number of moles, and temperature for any sample of any gas. The equation is PV = nRT.
0.13 mol
3.90 atm
Possible answers: Reduce the number of particles to 0.5 mol. Reduce the temperature to 137 K. Increase the volume to 44.8 L.
Lesson 66
Humidity is a measure of the number density of molecules of water vapor in the air.
about 1.7 mol per 1000 L
2.2 mol per 1000 L
0.43 mol per 1000 L
No, if the amount of water vapor in the air remains constant, it will still be less than the maximum possible vapor.
Yes, the amount of water vapor in the air cannot remain constant, because the maximum possible vapor at 20 ºC is less than the original amount of water vapor. Therefore, some water vapor will condense and form fog.
Lesson 67
Possible answer: For a hurricane to form, the temperature at the ocean’s surface must be at least 80 ºF and the air must contain a lot of moisture.
Chapter 12 Review Exercises
D
68%
Unit 3 Review Exercises
General Review
The ice has a lower mass because it has a lower density than liquid water. The density of water is 1.0 g/mL. Mass can be calculated by multiplying the density by the volume, and because the samples have the same volume, the ice has less mass.
According to the kinetic theory of gases, gas particles move faster when they have more energy. When a gas is heated, its particles gain energy, move faster, and scatter farther apart, causing the gas to expand. When the gas is cooled, its particles lose energy, causing them to move more slowly and remain closer together, so the gas contracts.
2.05 atm
273 K, 1.0 atm
1 mol
2 mol
0.0446 mol/L
12 mol
Standardized Test Preparation
| 1. A | 3. D | 5. B |
| 7. D | 9. C | 11. A |
| 13. D | 15. A | 17. C |
| 19. B |