Featured LAB: Thermometers
Featured LAB
Thermometers
Purpose
To examine how the volume of a liquid and a gas change in response to temperature.
Part 1: Liquid Thermometer

Procedure
Follow the handout instructions to build a thermometer like the one shown here. Then mark the liquid level in the straw for these conditions.
room temperature
vial warmed by your hand
ice water
ice water with 1 tablespoon of salt per 200 mL water
boiling water (do not allow the thermometer to touch the bottom of the beaker)
Observations and Analysis
What did you observe? What is happening to the liquid in the vial to make it move up and down in the straw?
Create a scale for the thermometer. Assign numbers for the places you marked on the straw for boiling water and ice water. What numbers did you choose and why?
Based on your newly created temperature scale, estimate the temperature in the room. How did you arrive at your answer?
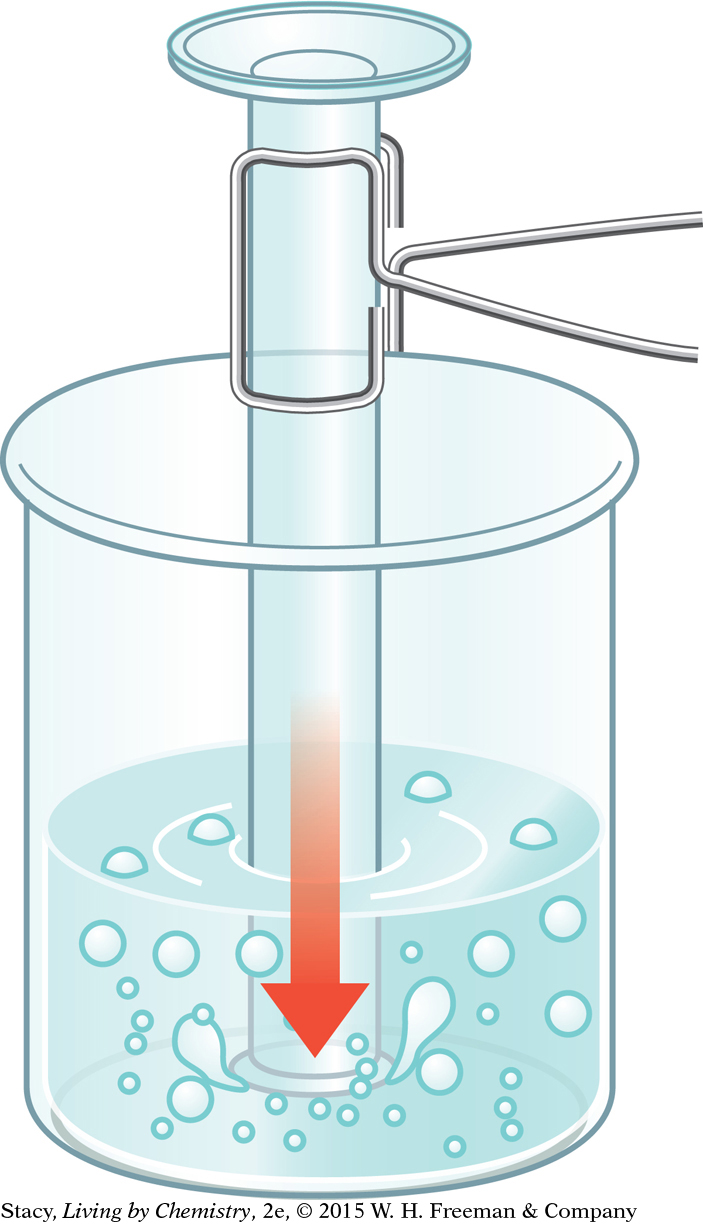
Part 2: Gas Thermometer
Materials
250 mL beakers (3)
ice
10 mL graduated cylinder
food coloring
test-tube holder—wire
hot plate
Procedure
Set up three beakers: one with ice water, one with room-temperature water, and one with hot water (cooled enough so that it is no longer steaming). Add 1–2 drops of food coloring to each beaker.
Hold the 10 mL graduated cylinder with a test-tube holder. Invert the graduated cylinder and immerse it in each beaker for one minute.
Making Sense
Describe how a thermometer works.