LESSON 53: Absolute Zero: Kelvin Scale
THINK ABOUT IT
HISTORY CONNECTION
HISTORY
CONNECTION
The French inventor and physicist Guillaume Amontons first proposed the existence of absolute zero in 1702. Although he became deaf in his early teens, he did not allow this to stop him from having a productive scientific career. In addition to his work on temperature and pressure, Amontons developed the gas thermometer.

The coldest temperature recorded on Earth was in Antarctica: a chilling –89 °C. The temperature is even lower on other planets that are farther away from the Sun. Researchers have recorded temperatures as low as –235 °C on the surface of Triton, a moon of Neptune!
How cold can substances become?
To answer this question, you will explore
Absolute Zero
Kelvin Scale
Molecules in Motion
Absolute Zero
EXPLORING THE TOPIC
Absolute Zero
The volumes of most substances decrease as the temperature decreases. But there is a limit to how much a substance can shrink. It does not make sense that matter would have a negative volume. Hypothetically, the lowest temperature possible would correspond to a volume of zero.
Consider an example. The volume of a gas inside a flexible container is measured as the gas is cooled. Several data points are then plotted on a graph.
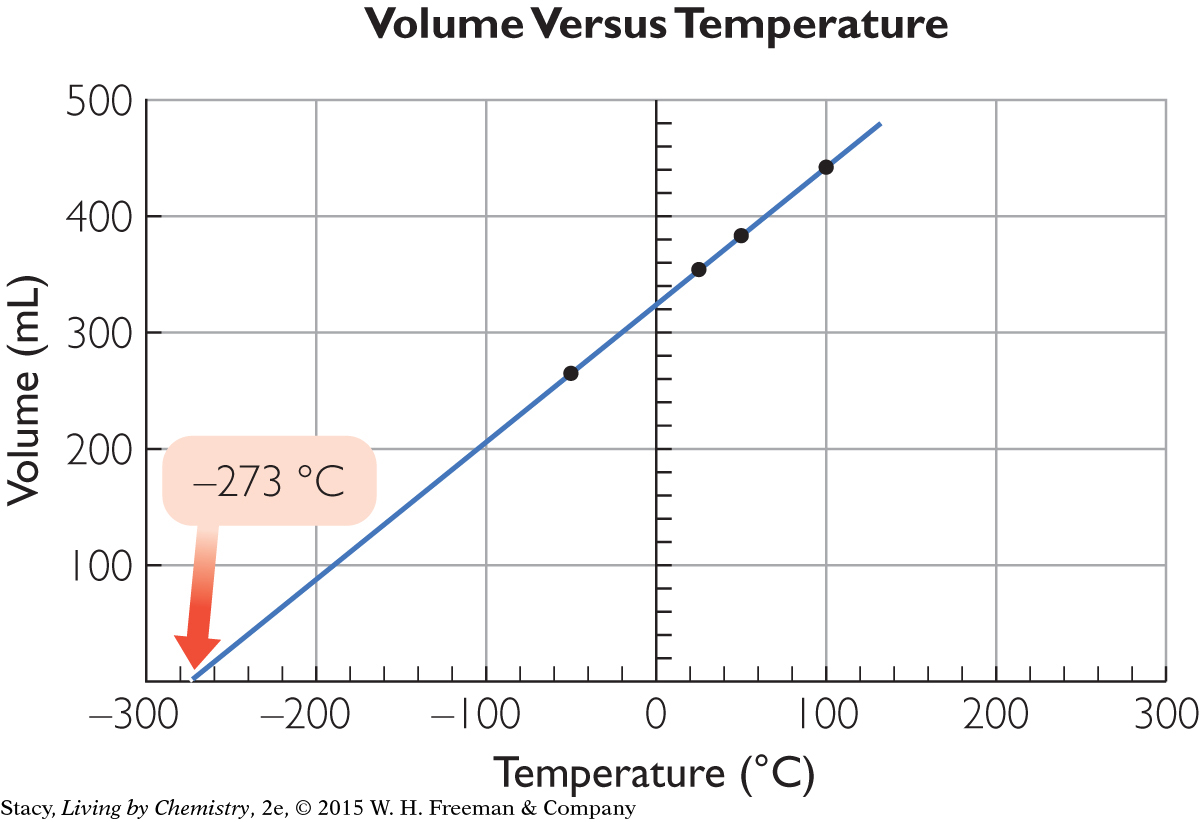
More precise measurements reveal –273.15 °C as the temperature at zero volume. Scientists hypothesize that this value corresponds to the lowest temperature possible, and call it absolute zero. In actuality, as the temperature is lowered, a gas would condense to a liquid and then to a solid well above this temperature, so zero volume is a property that is still a hypothesis.
Kelvin Scale
Kelvin Scale
On the Celsius scale, the temperature at which the volume of gas is theoretically zero is –273 °C. If you want to create a scale where the temperature is 0 when the volume is 0, you must add 273 to each temperature. This new temperature scale is called the Kelvin scale. One kelvin is the same size as one Celsius degree.
K = C + 273
C = K – 273
For example, to convert 20 °C to kelvins, add 273. This temperature corresponds to 293 K.
HISTORY CONNECTION
HISTORY
CONNECTION
The Kelvin temperature scale is named after William Thomson, 1st Baron Kelvin, also known as Lord Kelvin, who proposed the scale in 1848.
Note that the word degree, or the symbol for degree, °, is not used with the Kelvin scale. The unit of temperature on the Kelvin scale is the kelvin, K.
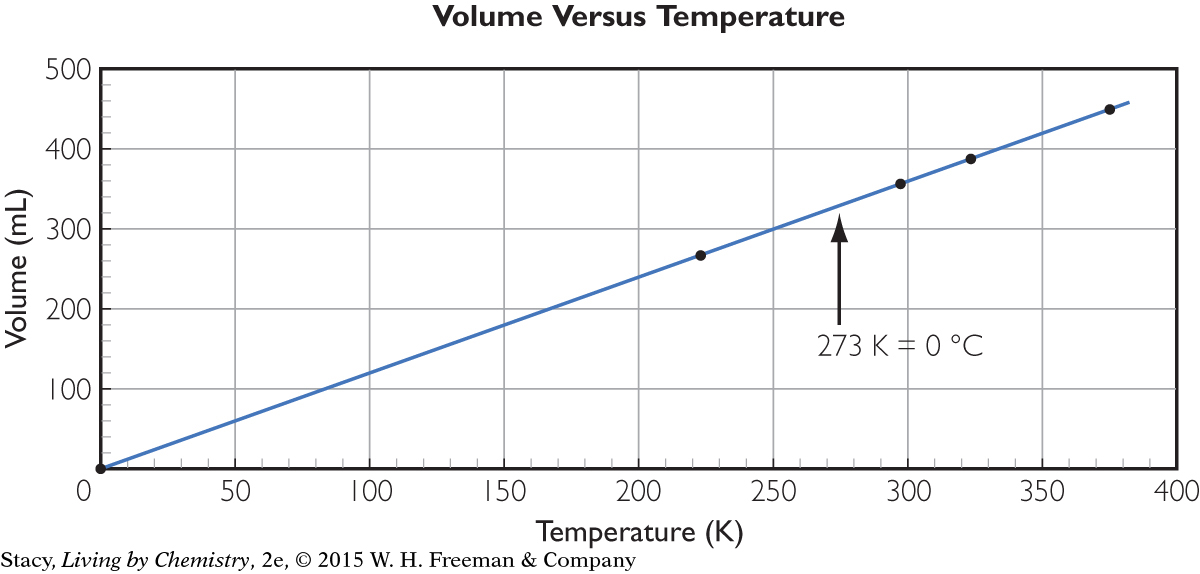
Room temperature is often considered to be 20 °C, or 68 °F, so 293 K is a temperature you will often find in problems. If you subtract 273 from a temperature in kelvins, you will be back at the Celsius temperature.
Molecules in Motion
Molecules in Motion
Gases are quite different from liquids and solids. The molecules and atoms in solids and liquids are held close together by forces between molecules and atoms. Liquids lie at the bottom of any container they are in, and solids generally maintain their own shape without a container. In contrast, the molecules in gases disperse to fill whatever space they are in. This indicates that there are no forces between the molecules in a gas.
ASTRONOMY CONNECTION
ASTRONOMY
CONNECTION
The Boomerang Nebula is the coldest place known in the universe. It is 5000 light-years from Earth in the constellation Centaurus and has a temperature of 1 K.

So, why is it that gas molecules are found throughout their container and not just at the bottom of it? And why is it that the space a gas occupies expands so dramatically as temperature increases? To explain many of the properties of gases, scientists rely on a model called the kinetic theory of gases. This model proposes that gas particles—atoms or molecules—are in constant motion. The word kinetic comes from a Greek word, kinetikos, meaning “moving.”
Big Idea
Big Idea
On a particle level, all matter is in constant motion.
These illustrations show the motion of three gas particles at three different times, based on the kinetic theory of gases.
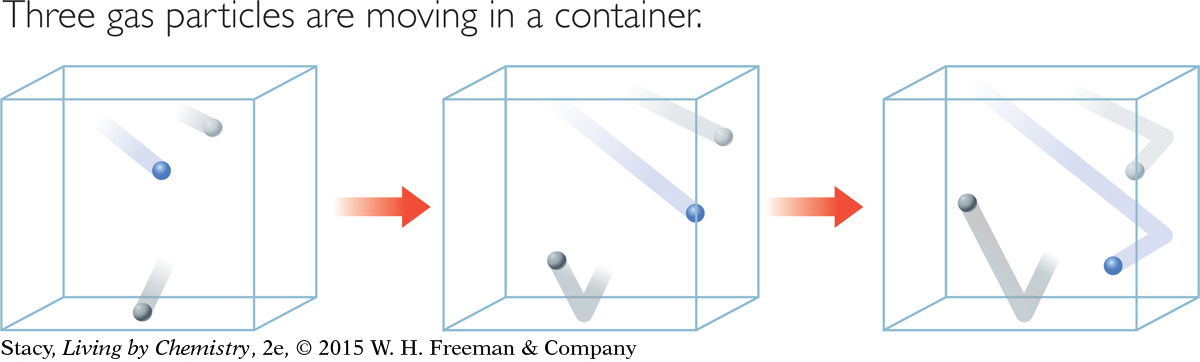
One characteristic of gases described by the kinetic theory is that not all gas particles are moving at the same speed. For example, the gas particle shown in blue moves more from frame to frame than the one shown in gray. However, the kinetic theory focuses on the average speed for all the particles at a given temperature. Indeed, the temperature of a gas can be defined as a measure of the average kinetic energy of the gas particles. When the temperature increases, the average speed of the gas particles increases. Scientists hypothesize that if you could cool matter to absolute zero, the atoms in the substance would stop moving.
Big Idea
Big Idea
Temperature is a measure of the average speed of the atoms or molecules in a sample.
LESSON SUMMARY
LESSON SUMMARY
How cold can substances become?
KEY TERMS
absolute zero
Kelvin scale
kinetic theory of gases
temperature
The Kelvin scale assigns a value of 0 K to the hypothetical temperature of a gas with zero volume. This point is called absolute zero and is at –273 °C. Scientists consider this to be the lowest hypothetical temperature that matter can reach. They hypothesize that all motion stops at absolute zero. The kinetic theory of gases describes the motions of the gas particles. The atoms or molecules in a gas are constantly moving at an average speed that increases with increasing temperature.
Exercises
Reading Questions
What is absolute zero? Why is it considered a hypothetical temperature?
What advantages does the Kelvin scale have over the Celsius scale?
How does the kinetic theory of gases explain temperature?
Reason and Apply
What are the freezing and boiling temperatures of water in degrees Celsius, kelvins, and degrees Fahrenheit?
Which unit is the smallest: one Celsius degree, one kelvin, or one Fahrenheit degree? Explain your thinking.
Would you describe each of these temperatures as warm, hot, or cold?
100 K
60 °C
250 K
25 °C
300 K
–100 °C
400 K
Convert each of the Kelvin temperatures in Exercise 6 to degrees Celsius, and vice versa.
What do you think is the highest temperature that can be reached by a substance? What is your reasoning?
Here are a few common temperatures on the Fahrenheit scale. Convert each of these to the Kelvin scale.
95 °F (a hot day)
350 °F (oven temperature)
5 °F (freezer)
The temperature on the surface of Venus is 736 K. Convert this temperature into degrees Fahrenheit and degrees Celsius. Describe what the atmosphere of Venus might be like.
Choose the best answer. According to the kinetic theory of gases, particles in a sample of gas
Move randomly, in curved paths, at different speeds, and bounce off each other and the walls of their container.
Move randomly, in straight-line paths, at different speeds, and bounce off each other and the walls of their container.
Move randomly, in straight-line paths, at the same speed, and bounce off each other and the walls of their container.
Move randomly, in curved paths, at the same speed, and bounce off each other and the walls of their container.
Use the kinetic theory of gases to explain why gases expand upon heating and shrink upon cooling.