LESSON 58: Feeling under Pressure
304
THINK ABOUT IT
SPORTS CONNECTION
SPORTS
CONNECTION
The basketball used by professional players is inflated to a pressure sufficient to make it rebound to a height of 5.4 feet when it is dropped from a height of 6 feet. This test is conducted in a basketball factory, and then that air pressure is stamped on the outside of the ball.

A flat basketball does not bounce very well. However, you can push air into the ball with a pump. The air you trap inside pushes on the skin of the ball with a lot of pressure. The amount of air you push into the ball determines how firm and bouncy the ball is. What would happen if you pushed the same amount of air into a smaller ball?
How does gas volume affect gas pressure?
To answer this question, you will explore
The Syringe and Scale
Graphing Pressure–Volume Data
Boyle’s Law
The Syringe and Scale
EXPLORING THE TOPIC
The Syringe and Scale
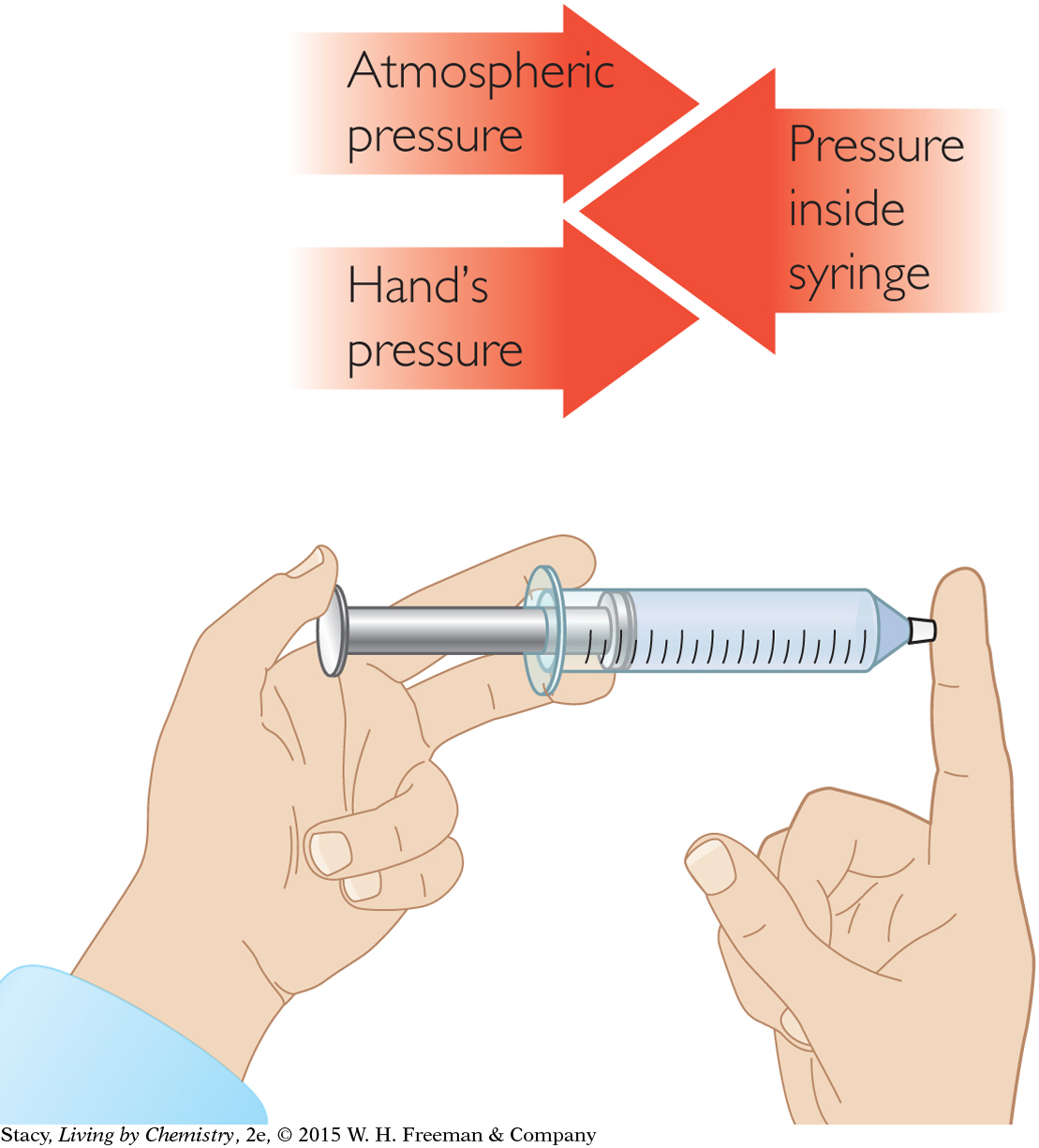
In class, you examined how the pressure of a gas trapped inside a container is related to the volume the gas occupies. A syringe makes a good container for such an investigation, because it can be sealed off and the volume of air trapped inside the syringe can be measured.
If you push down on the plunger of the syringe, you can feel the pressure of the gas inside the syringe. Air molecules take up space and as you squeeze them into a smaller space, they push back more and more. This is because the same number of molecules occupies a smaller volume, resulting in more collisions between the molecules and the container. More collisions mean greater gas pressure.
GATHERING DATA
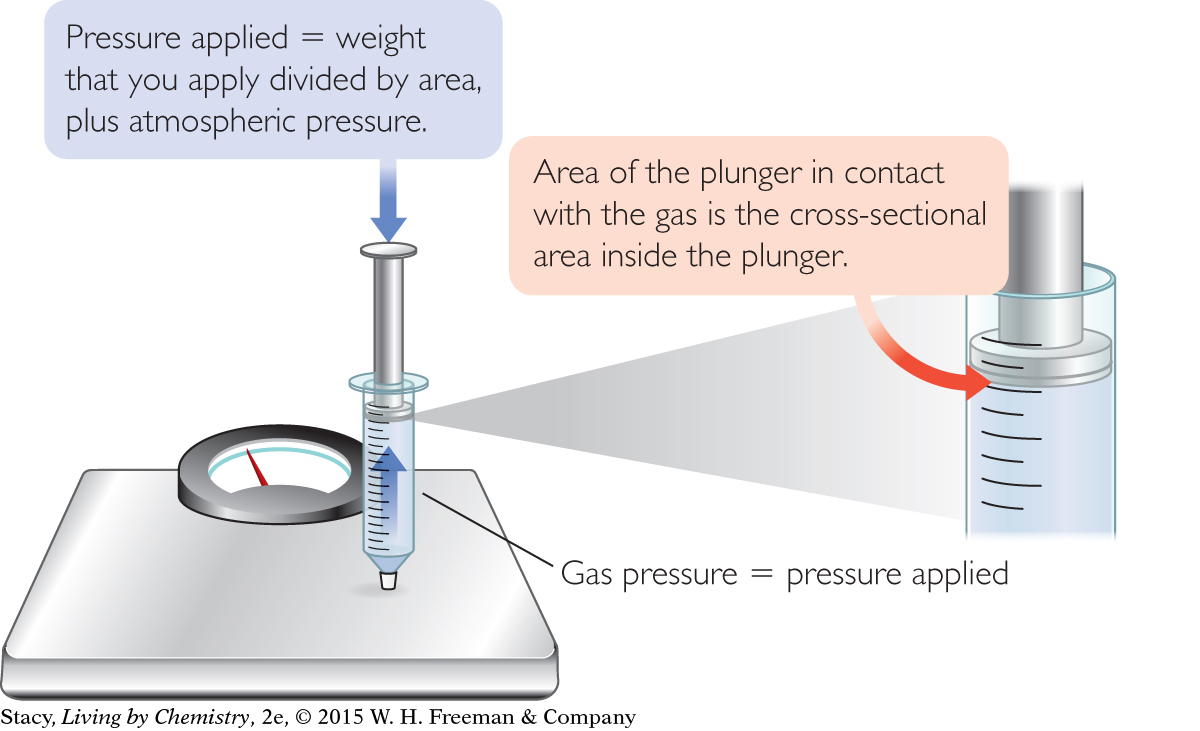
You can learn more about the relationship between the volume of a gas and its pressure by using a capped syringe and a bathroom scale. As you push down on the plunger of the syringe, the gas pressure inside the syringe increases, and the weight on the scale increases. You can record this quantitative, or numerical, data. To calculate the pressure of the gas, divide the measured weight on the scale by the surface area of the plunger in pounds per square inch, or lb/in2. Weight is a measure of force.
305
Important to Know
Because the atmosphere is also pushing on the syringe, you need to add 14.7 lb/in2 to the pressure that you apply.
Note that the pressure you apply plus atmospheric pressure is equal to the gas pressure inside the syringe. When you push down, the gas pushes back with the same pressure that is being applied to it.
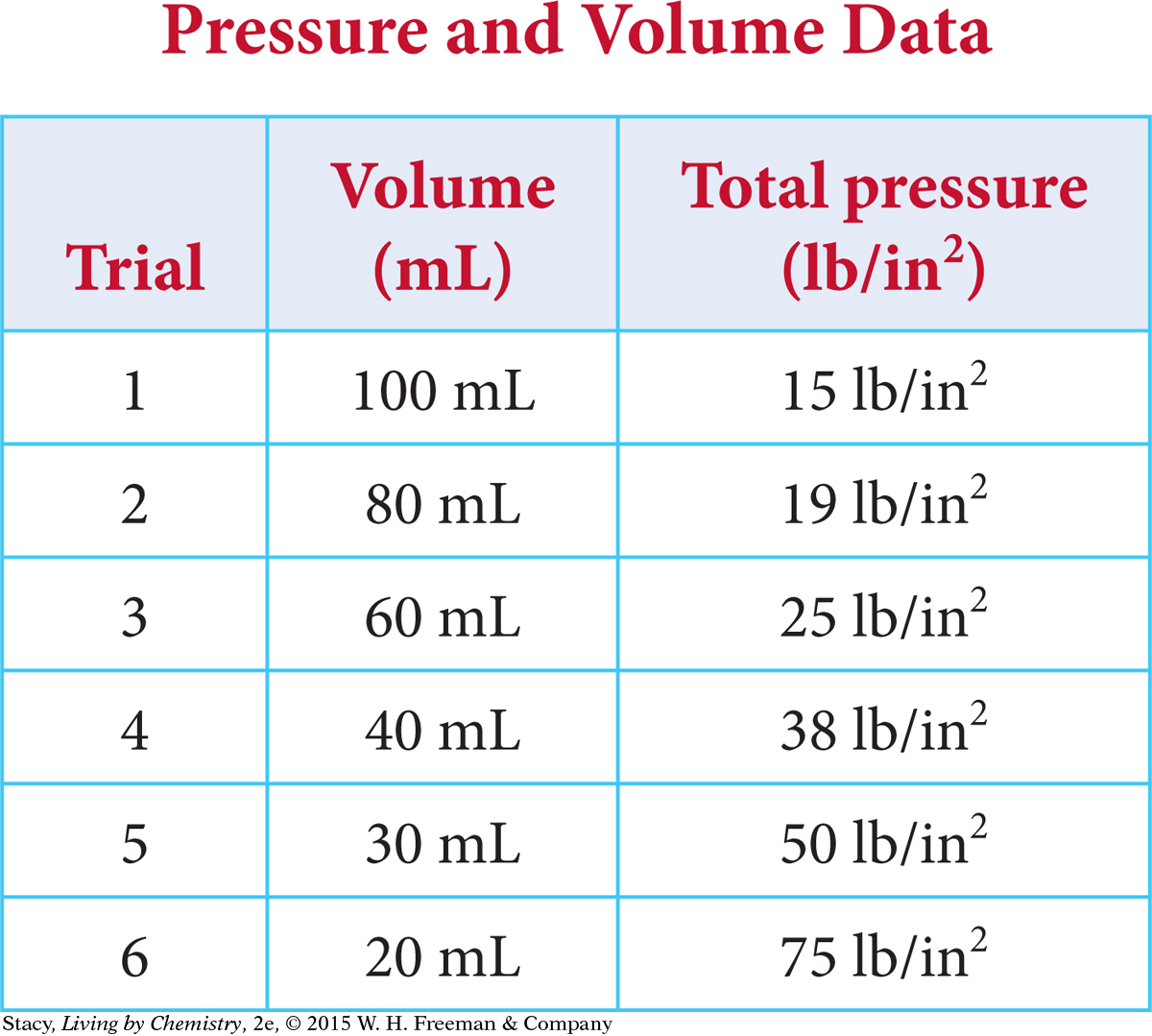
Data for this experiment are given in the table. Notice that as the volume decreases, the pressure of the gas inside the syringe increases.
Big Idea
Big Idea
If you squeeze a sample of gas into a smaller container, its pressure will increase.
Graphing Pressure–Volume Data
Graphing Pressure–Volume Data
Many of the relationships you have explored so far, like mass versus volume and volume versus temperature of a gas, are proportional relationships. The relationship between pressure and volume is not directly proportional. A graph of the data for this experiment results in a curved line that does not go through the origin.
306
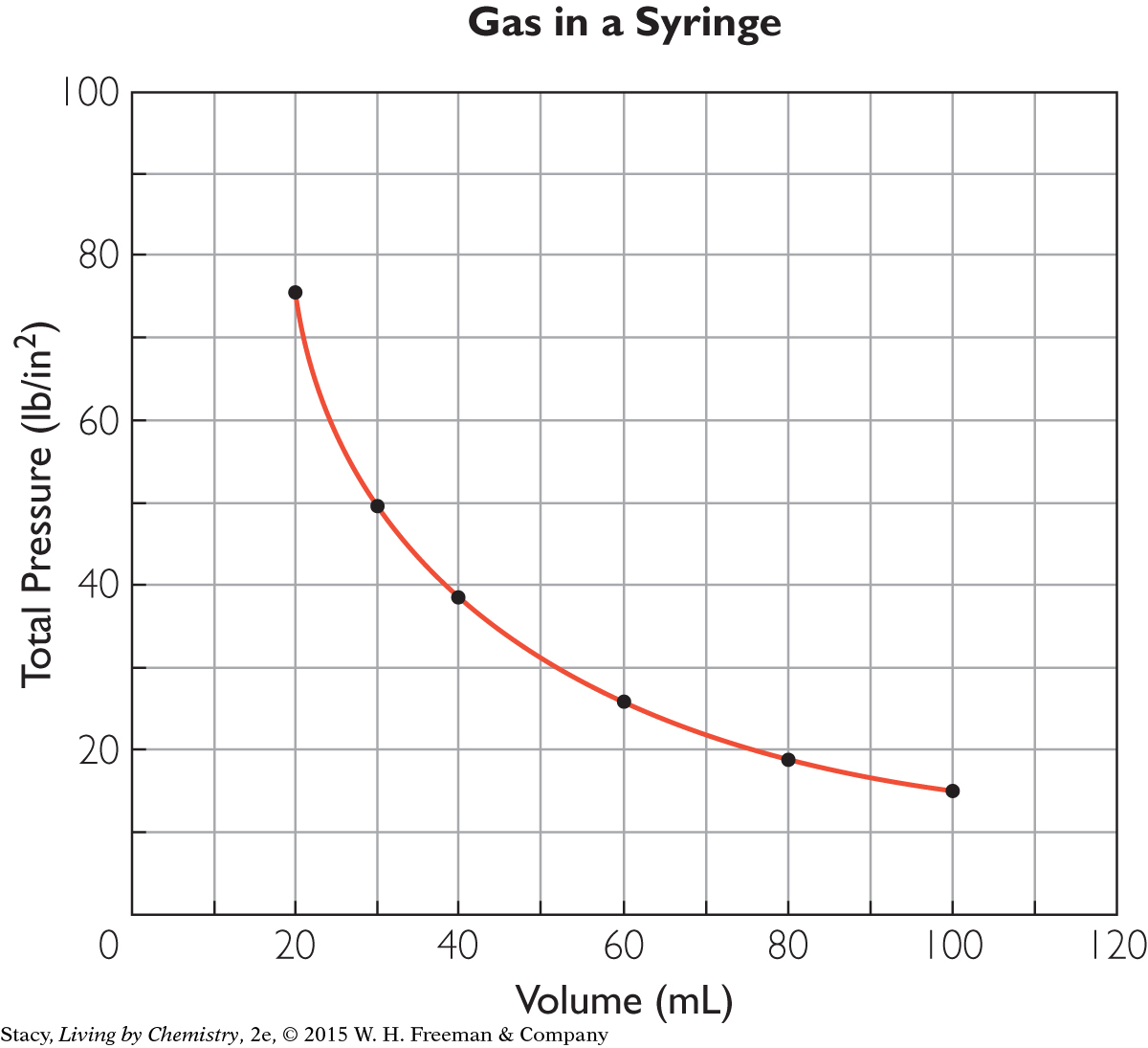
Gas pressure and gas volume are inversely proportional to one another. In other words, when one variable increases, the other decreases, and vice versa.
When the volume of the gas in the syringe is very small, the gas pressure is quite high. When the volume of the gas is large, the gas pressure is quite low. However, as long as there are gas molecules in a container, neither the gas pressure nor the gas volume can ever reach zero.
Boyle’s Law
Boyle’s Law
In 1662, a British scientist named Robert Boyle discovered that gas pressure and volume are inversely proportional to each other. This relationship is known as Boyle’s law.
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Gases are often stored in tanks at high pressure or even at low temperatures so that they’re liquid, because otherwise the containers needed for them would be huge. These tanks are usually made of thick metal so that they don’t change volume.

Boyle’s Law
The pressure P of a given amount of gas is inversely proportional to its volume V, if the temperature and amount of gas are not changed. The relationship is
PV = k or 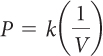 , where k is the proportionality constant.
, where k is the proportionality constant.
Examine the Pressure and Volume Data table on page 305. If you multiply pressure by volume, the product will always be around 1500. (It will vary slightly due to rounding and slight errors in measurement.) Boyle’s law expresses this relationship as PV = k. In this experiment, the proportionality constant, k, is 1500 mL · lb/in2. [For a review of this math topic, see MATH Spotlight: Ratio and Proportions on page A-11.]
Boyle’s law can be used to solve problems involving gas pressure and gas volume. Once you have one set of pressure and volume measurements for a gas, you can determine the proportionality constant. With the proportionality constant, you can determine the pressure of the gas at any volume.
307
Example
Gas in a Syringe
Determine what the pressure of the gas inside this syringe would be if the volume were reduced to 10 mL.
Solution
|
Start with Boyle’s law. |
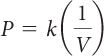
|
|
Substitute values for k and V. |
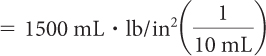
|
|
Solve. |
= 150 lb/in2 |
You can check this answer: PV = 150 lb/in2 · 10 mL = 1500 mL · lb/in2.
LESSON SUMMARY
LESSON SUMMARY
How does gas volume affect gas pressure?
KEY TERMS
inversely proportional
Boyle’s law
If you squeeze a gas into a smaller volume, it will exert more pressure on its container because a smaller space means more collisions between the molecules and the container. If you let a gas spread out into a huge volume, the gas pressure will get very low because there will be fewer collisions between the molecules and the container. Gas pressure and gas volume are inversely proportional. This means that as one variable increases, the other decreases, and vice versa. This relationship is described by Boyle’s law: P = k(1/V).
Exercises
Reading Questions
Explain how you can use a scale to measure the pressure of the gas in a syringe.
Describe how pressure varies as the volume of a trapped gas changes.
Reason and Apply
Lab Report Write a lab report for the Lab: Boyle’s Law. In the results section, provide two graphs of the data. Plot pressure versus volume and plot pressure versus the inverse of the volume, 1/V. Also discuss how accurate your results are and what factors might contribute to experimental error in this lab. In the conclusions section, explain the relationship between pressure and volume. Explain how your data relate to Boyle’s law.

308
You are using a bicycle pump to fill a bicycle tire with air. It gets harder and harder to push the plunger on the pump the more air is in the tire. Explain what is going on.
How is the relationship between gas pressure and gas volume different from the relationship between gas volume and gas temperature?
Imagine you blow up a balloon before going scuba diving. You put on your gear and descend to 30 ft with the balloon. The total pressure from the ocean at that depth is 2 atm. Assume temperature is constant.
What happens to the volume of the balloon?
What happens to the pressure of the air on the inside of the balloon?
This table shows experimental data for gas volume and pressure.
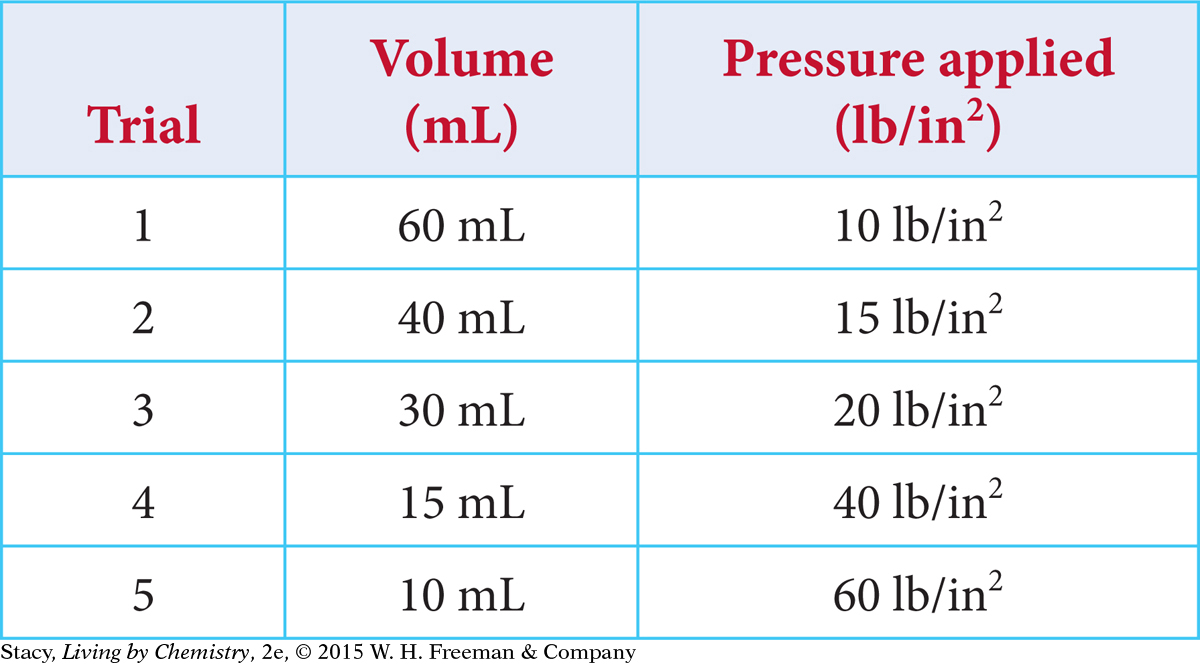
Show that P = k(1/V) for each trial. Remember to add 14.7 lb/in2 to the values in the last column to account for atmospheric pressure.
Show that PV = k for each trial.
Create a graph of P versus V. What does the graph tell you about the relationship between gas pressure and volume?
Create a graph of P versus 1/V. What does the graph tell you about the relationship between gas pressure and inverse volume?
Use the graphs from parts c and d to estimate the volume when the pressure is 30 lb/in2.
Use the graphs from parts c and d to estimate the pressure when the volume is 50 mL.