LESSON 70: Spare Change: Physical versus Chemical Change
363
THINK ABOUT IT
You breathe in oxygen and it dissolves in your blood, where it binds to a molecule called hemoglobin. The hemoglobin transports the oxygen to where it is needed for reactions with carbohydrates. In the end, the oxygen and carbohydrates react to produce carbon dioxide and water. Some of these changes are physical changes and some are chemical changes.
How are changes in matter classified?
To answer this question, you will explore
Defining Physical and Chemical Change
Dissolving: A Special Case
Defining Physical and Chemical Change
EXPLORING THE TOPIC
Defining Physical and Chemical Change
Examine the chemical equations in the table.
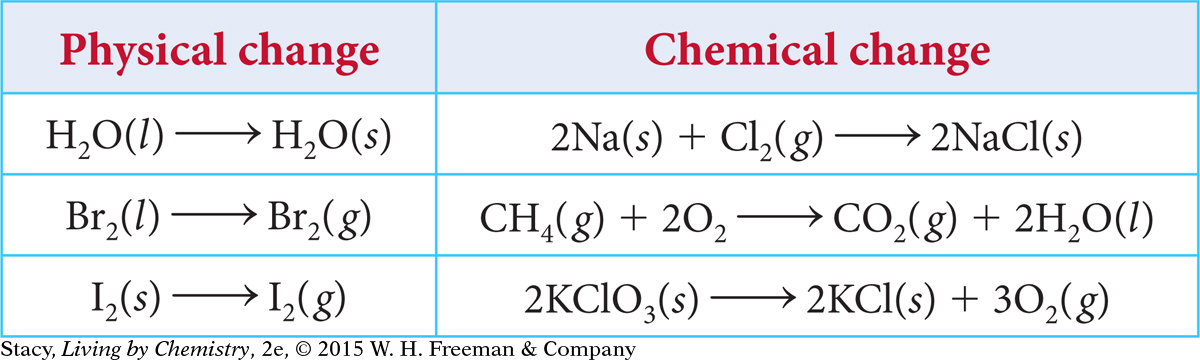
PHYSICAL CHANGE
The changes in the left column are all physical changes. In each case, the substance on the left side of the equation changes phase (solid, liquid, or gas), but it does not change its identity. Also, there is only one substance involved in each of these equations, and that one substance is present on both sides of the arrow.

H2O(l) → H2O(s)
Liquid water freezes to become ice.
Brian Mueller/Shutterstock
|

Br2(l) → Br2(g)
Liquid bromine evaporates to bromine gas.
Charles D. Winters/Science Source
|

I2(s) → I2(g)
Solid iodine sublimes to iodine gas.
Ken Karp Photography
|
364
When something changes in a physical way, the chemical formula does not change because you end up with the same substance you started with. Changing pressure or temperature, and grinding a substance into a powder, are other examples of physical change.
CHEMICAL CHANGE
The three equations in the right column of the table on the previous page are chemical changes. In each case, new substances are created. In the equations, the chemical formula(s) on the left side of each arrow are different from the formula(s) on the right side. New substances are formed during chemical changes, so we can expect the products to have properties significantly different from the reactants.

2Na(s) + Cl2(g) → 2NaCl(s)
Solid sodium reacts with chlorine gas to produce solid sodium chloride.
Andrew Lambert Photography/Science Source
|

CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
Methane gas and oxygen gas burn to produce carbon dioxide gas and water.
vesilvio/Shutterstock
|
2KClO3(s) → 2KCl(s) + 3O2(g)
Solid potassium chlorate is heated to form solid potassium chloride and oxygen gas.
Philip Evans
|
Heat, light, smoke, smells, bubbles, and changes in color often accompany chemical changes. When chemical change takes place, chemists say that a chemical reaction has occurred. In fact, the terms chemical change and chemical reaction mean the same thing.
Dissolving: A Special Case
Dissolving: A Special Case
ART CONNECTION
ART
CONNECTION
Many ancient buildings and monuments such as the Great Sphinx in Egypt, shown here, are being destroyed by acid rain because they are made of materials like limestone that dissolve in sulfuric acid.

Chemical equations can also represent the process of dissolving. Here are three equations that represent dissolving.
Liquid ethanol dissolves in water: C2H6O(l) → C2H6O(aq)
Gaseous hydrochloric acid dissolves in water: HCl(g) → HCl(aq)
Solid copper chloride dissolves in water: CuCl2(s) → CuCl2(aq)
These equations indicate that the identity of each dissolved substance has not changed. Each equation describes a physical change. The symbol (aq), for aqueous, on the product side of the equation means that each substance has dissolved in water.
Here is a different way to write the second and third equations listed above.
HCl(g) → H+(aq) + Cl–(aq)
CuCl2(s) → Cu2+(aq) + 2Cl–(aq)
Important to Know
You cannot always tell by observing a change whether it is a physical change or a chemical change. A chemical equation can provide more information than observation alone.
365
When hydrogen chloride gas and solid copper chloride dissolve in water, they break apart into ions. The second type of equation shows what happens during this change. These equations showing a substance breaking apart to form ions are necessary for the dissolving of ionic compounds and acids. Note that molecular substances like ethanol, C2H6O, do not break apart when they dissolve. So there is only one way to write the equation.
Ionic compounds dissolved in water have different properties from ionic solids. For example, ionic solutions conduct electricity, but the solid forms do not. This makes dissolving seem like a chemical change. However, the identity of the substances has not changed. You can recover solid compounds from solutions by evaporating the water they are dissolved in. So you can categorize dissolving as either physical or chemical change.
Example
Magnesium Chloride and Water
Write two equations that show what happens when magnesium chloride, MgCl2(s), dissolves.
Solution
MgCl2(s) → MgCl2(aq)
MgCl2(s) → Mg2+(aq) + 2Cl–(aq)
LESSON SUMMARY
LESSON SUMMARY
How are changes in matter classified?
KEY TERM
physical change
In general, changes in matter can be classified as either physical or chemical. Physical changes, including phase changes, involve a change in form without changing the identity of the substance. There is no change in chemical formula. Chemical changes, also called chemical reactions, involve the formation of new substances with new properties. The chemical formulas of these new products are different from the chemical formulas of the reactants. Dissolving can fall into both categories of change, though it is usually considered a physical change.
Exercises
Reading Questions
What is the difference between a physical change and a chemical change?
Explain why dissolving can be described as either a physical or chemical change.
Reason and Apply
Give five examples of physical changes. Explain why each example is a physical change.
366
Copy the equations 1–7 onto your paper. Match the chemical equations with their descriptions.
2CH4O(l) + O2(g) → 2CH2O(l) + 2H2O(l)
NH2Cl(g) → NH2Cl(aq)
2C8H14(l) + 23O2(g) → 16CO2(g) + 14H2O(l)
H2SO4(aq) + CaCO3(s) → CaSO4(aq) + CO2(g) + H2O(l)
Hg(l) → Hg(g)
C16H30O2(s) + H2 → C16H32O2(s)
CaO(s) + H2O(l) → Ca(OH)2(s)
The sulfuric acid dissolved in raindrops of acid rain reacts with the calcium carbonate in seashells and marble structures. This reaction produces calcium sulfate dissolved in water and carbon dioxide gas.
To balance the effect of acid rain, solid calcium oxide has been added to many lakes. It reacts with water to form solid calcium hydroxide, a strong base that is only slightly soluble in water.
Chloramine is added to our water supply in very small amounts to kill bacteria.
When octane and oxygen gas are burned in our cars, carbon dioxide and water come out in the exhaust.
Methanol, if ingested, reacts with oxygen to form formaldehyde, which is toxic. Water is also formed in this reaction.
Liquid mercury evaporates to produce mercury vapor.
Saturated fatty acids, like palmitic acid, tend to form long solids and clog people’s arteries. These saturated fatty acids can be made from unsaturated fatty acid by adding hydrogen gas.
For equations 1–7 from Exercise 4, label each one as a physical or chemical change.
Classify the following two changes as physical or chemical. Explain your reasoning.
CaCO3(s) + H2SO4(aq) → CaSO4(aq) + CO2(g) + H2O(l)
NaCl(s) → NaCl(l)