Chapter 14 Summary

CHAPTER 14
Measuring Toxins
SUMMARY
KEY TERMS
toxicity
percent error
molar mass
scientific notation
Toxins Update
To keep the public safe, health experts measure the toxicity of substances that people might be exposed to in the course of their daily lives. The most common measurement of toxicity is called lethal dose, or LD50, which is expressed in milligrams per kilogram of body weight. The higher the LD50 for a substance, the safer the compound is.
Chemical formulas track compounds in counting units called moles. To determine if a certain quantity of matter amounts to a lethal dose, it is necessary to convert between mass and moles using molar mass.
REVIEW EXERCISES
Question 14.1
1. Mass and moles are proportionally related. Explain what this means.
Question 14.2
2. Why is it necessary to take a person’s weight into account when considering the correct dose for a medication?
Question 14.3
3. Why is scientific notation a useful tool for chemists?
Question 14.4
4. Consider vitamin D3, C27H44O, cholecalciferol. Vitamin D3 helps the body extract calcium and phosphorus from food, plays a role in mental health, and may prevent some cancers. Most of the vitamin D you get is produced in your skin when you are exposed to the Sun.
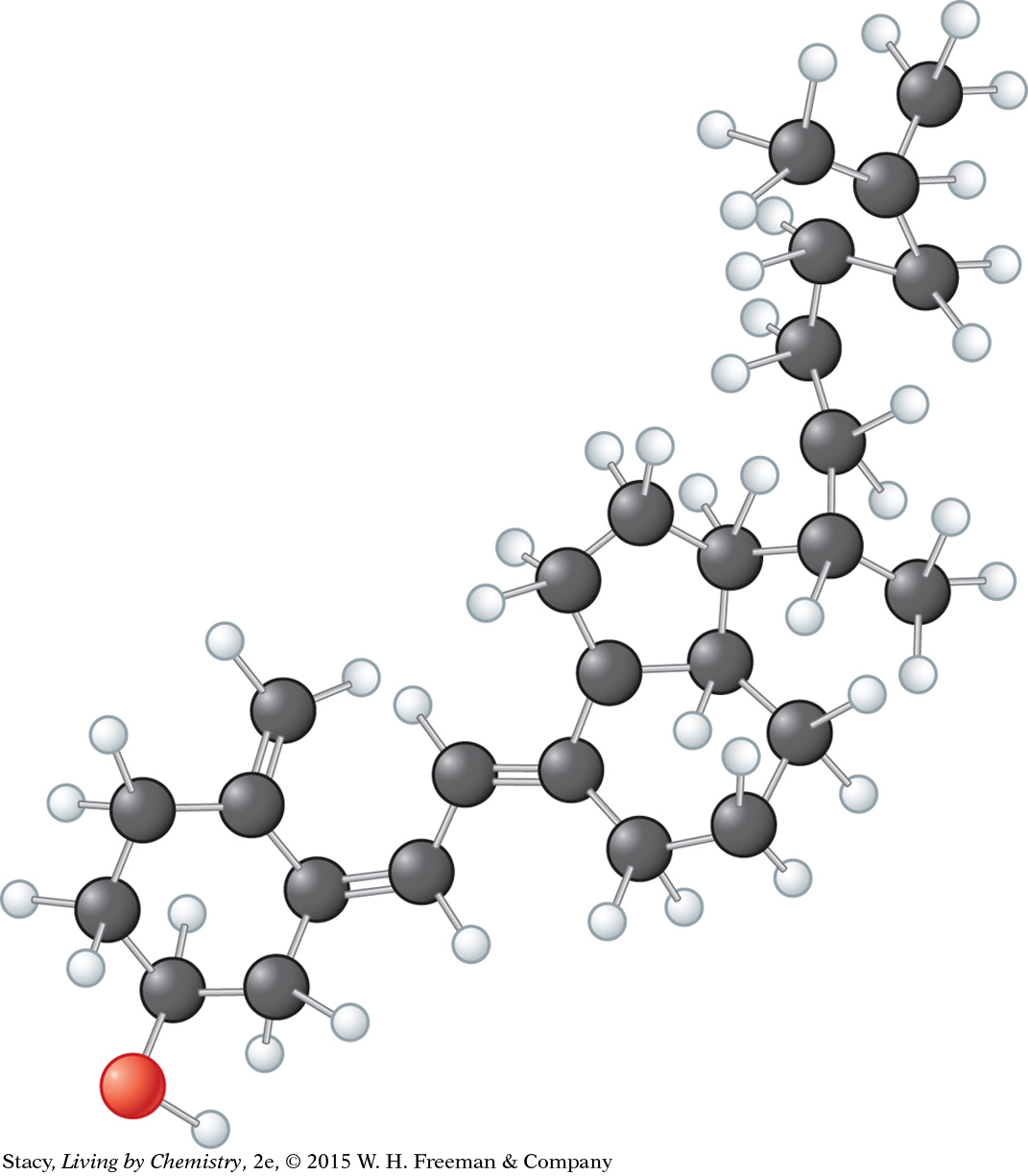
Lethal dose: LD50 = 42.0 mg/kg (rat, oral)
Recommended Dietary Allowance: RDA = 0.010 mg/day
Determine the number of moles and molecules of vitamin D3 in the Recommended Dietary Allowance.
Determine the lethal dose for a 140 lb person.
Determine the number of moles of vitamin D3 in a lethal dose for a 140 lb person.
Determine the number of molecules of vitamin D3 in a lethal dose for a 140 lb person.
Would 1.0 × 1020 molecules of vitamin D3 represent a lethal dose for a 140 lb person? Explain your reasoning.
Would 400 tablets (0.010 mg each) of vitamin D3 be a lethal dose for a 140 lb person? Explain your reasoning.
Question 14.5
5. List these compounds in order of increasing moles of metal in each compound: 5.0 g NaCl, 5.0 g AgCl, 5.0 g LiCl. Show your work.
Question 14.6
6. Copy and balance these chemical equations.
TiCl4(s) + Mg(s) → Ti(s) + MgCl2(s)
Mg(s) + H2O(l) → MgO(s) + H2(g)
Fe(s) + CuSO4(s) → FeSO4(s) + Cu(s)
Li(s) + H2O(l) → LiOH(aq) + H2(g)