LESSON 75: Make It Count: Counting by Weighing
THINK ABOUT IT
When a toxin enters your body, it is important for a doctor to know exactly how much toxin there is. One molecule of snake venom has over 100 times as much mass as one atom of arsenic. So, 1 milligram of snake venom contains many fewer particles than 1 milligram of arsenic. To track the toxin, you need an inventory, or a count, of the atoms or molecules. However, atoms are so small that you cannot see them, let alone count them. Luckily, there are other ways of figuring out the number of atoms in a sample besides counting them one by one.
How can mass be used to count large numbers of small objects?
To answer this question, you will explore
Using Mass to Count
Weighing Atoms
Using Mass to Count
EXPLORING THE TOPIC
Using Mass to Count
Let’s explore how to count objects by weighing them. The masses of a number of different small objects are provided in the table.
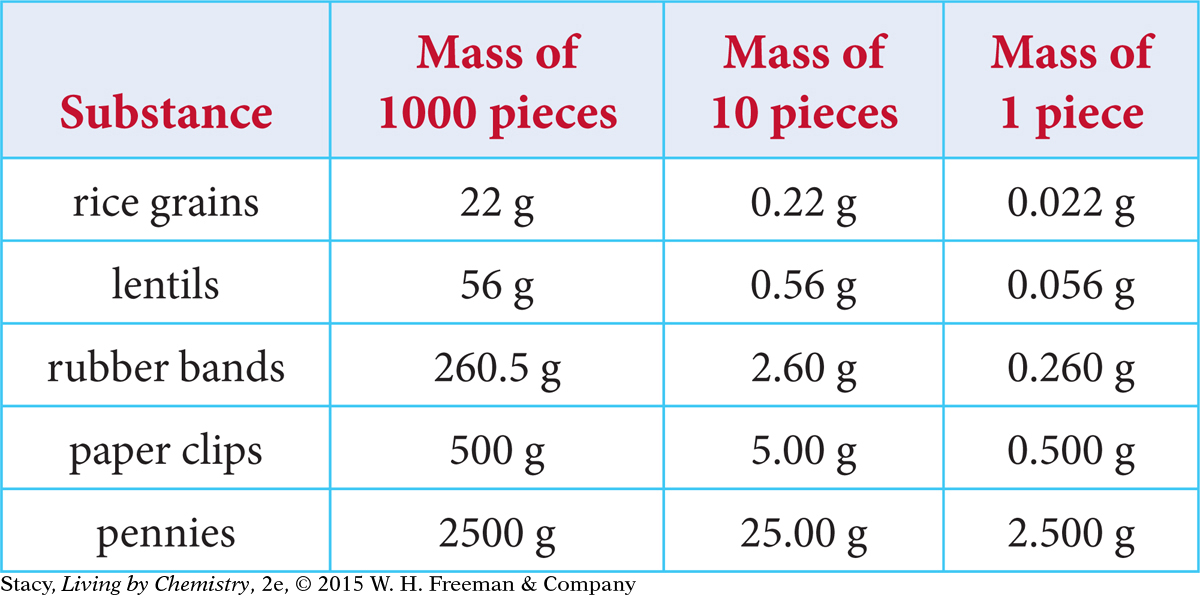
It is difficult to accurately measure the mass of an object if the object has a mass that is below the detection limit of the balance. Many electronic balances have 0.001 g as a lower weight limit. So, weighing heavier objects tends to yield more accurate results.
One way around this difficulty is to count out a collection of identical (or nearly identical) objects and weigh the collection. You can then find the mass of a single object by dividing the mass of the collection by the number of objects. This gives you an average mass of one object that is more accurate than if you had weighed the object individually.
Once you know the average mass of a single object, you can use it to determine exactly how many objects you have in a large sample. Consider a sample of pushpins.
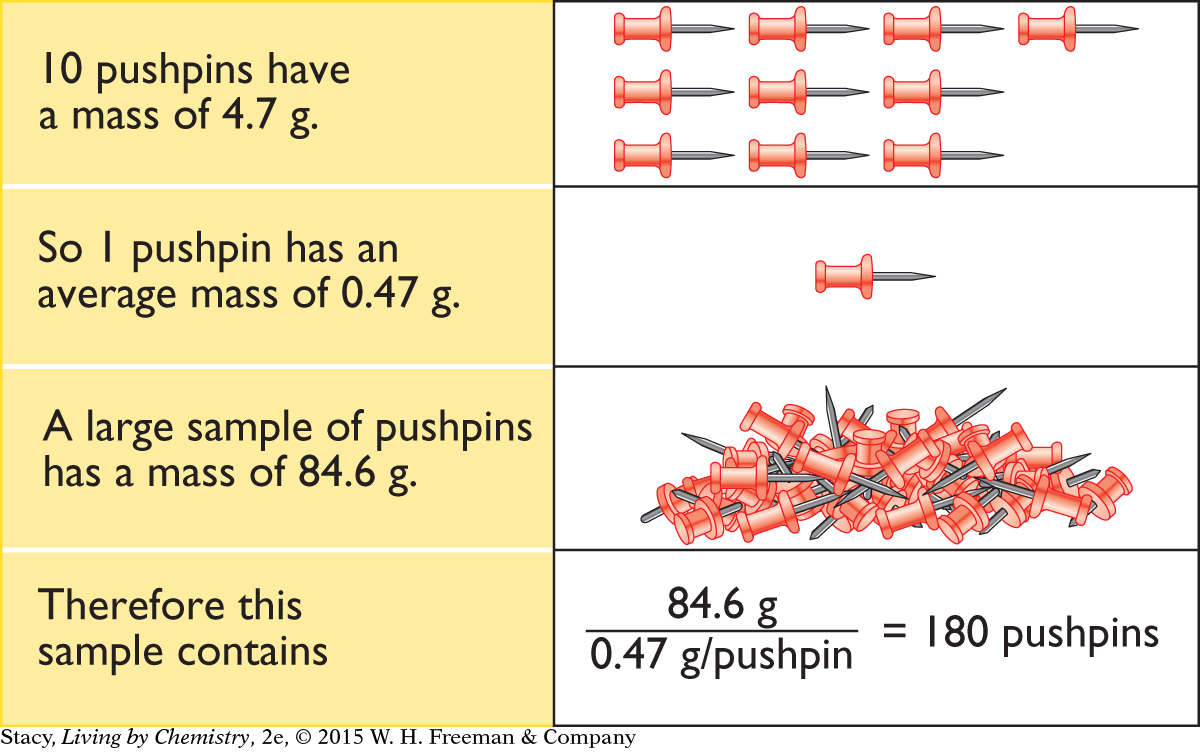
In addition to helping you overcome the detection limit of the balance, there is a second reason that it is important to find the average mass of the objects in a sample: There is a slight variation in the masses of the objects. For example, not all grains of rice are identical in size. Some are slightly larger and others are slightly smaller (like isotopes of atoms). So, an average mass is a more accurate predictor of the mass of a typical object.
Example
A 5 lb Bag of Rice
How many grains of rice are there in a 5 lb bag of rice? The average mass of a rice grain is 0.0221 g, and the bag contains exactly 5.00 lb. (1 lb = 454 g)
Solution
First, determine how many grains of rice are in the bag.
| Multiply by the conversion factor. |
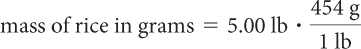
|
| = 2270 g rice |
This is the mass of all the grains of rice. Next, determine how many grains of rice are in the bag.
Total mass of rice = average mass of 1 grain · number of grains
| Divide the total mass of the rice by the average mass of 1 grain. |
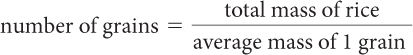
|
| Calculate the answer. |
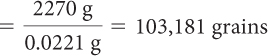
|
So, rounded to three significant digits, there are about 103,000 grains.
[For a review of these math topics, see MATH Spotlight: Dimensional Analysis on page A-16 and MATH Spotlight: Accuracy, Precision, and Significant Digits on page A-1.]
PERCENT ERROR
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Some snakebites can be harmless, others lethal. For example, the bites of garter snakes, found throughout North America, are generally harmless. However, it would take just 1/14,000 ounce of venom from an Australian brown snake, shown here, to kill a person.

Chemists use percent error to express how accurate their measurements are. For example, imagine a sandwich bag contains exactly 340 beans. Suppose you calculated the number of beans using mass and came up with 352 beans. To figure out your percent error, you would use this formula:
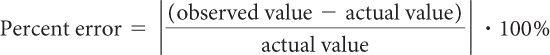
In this case, the percent error is
Notice that saying your answer was off by 3.5% is more meaningful than saying you were off by 12 beans, which could be a lot or a little, depending on how many beans you were counting. The smaller the percent error, the more accurate your answer.
Weighing Atoms
Weighing Atoms
You can apply the same method you used to count tiny objects to counting atoms. To determine the number of atoms in a 1.0 g sample, you will need to know the average mass of the atoms in the sample. The average mass of the atoms of each element is given on the periodic table in atomic mass units, or amu.
Atomic mass units are special units used for atoms because the mass of an atom in grams is so tiny. Each atomic mass unit is equal to 0.00000000000000000000000166 g. There are 23 zeros in front of the 166, making this number inconvenient to use.
Consider hydrogen atoms. Hydrogen has an average atomic mass of about 1.0 amu, or 0.00000000000000000000000166 g. How many hydrogen atoms are in a 1.0 g sample?
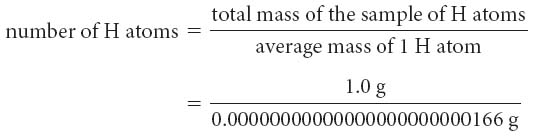
So 1 gram of hydrogen atoms contains 602 sextillion, or 1 mole, of hydrogen atoms.
LESSON SUMMARY
LESSON SUMMARY
How can mass be used to count large numbers of small objects?
KEY TERM
percent error
It is difficult to count very small objects like atoms and molecules. One method for getting a count of small objects is to weigh a large number of the objects and divide by the average weight of one object. The average atomic mass on the periodic table is equivalent to the mass of one atom of each element. This number is an average because some atoms are isotopes with different masses. The mass of a single atom is expressed in atomic mass units rather than grams. To find the number of atoms in a sample of an element, you can divide the mass of the sample by the average mass of one atom.
Exercises
Reading Questions
Explain how you can use mass to count large numbers of objects.
What does the percent error tell you?
Reason and Apply
Recall the method you used to count the objects in the sandwich bag you received in class and the results you obtained.
Explain what you were trying to find out.
Explain the method you used.
Show your calculations and results.
Calculate the percent error. Explain how you might modify your procedure to reduce the percent error.
Suppose that you have 50 grams of rice and 50 grams of beans. Which sample has more pieces? Explain your thinking.
Suppose that you have 740 tiny plastic beads and 740 marbles. Which sample has more mass? Explain your reasoning.
One bean weighs 0.074 g. How many beans are in a 50-pound bag?
Suppose you want to fill a 500 g bag with twice as many red jelly beans as yellow. What mass of red jelly beans do you need? A jelly bean weighs 0.65 g.
What is the mass in grams of one copper atom?
What is the mass in grams of one gold atom?
Suppose you have 50 grams of copper and 50 grams of gold. Which sample has more atoms? Explain your thinking.
Suppose you use mass to calculate the number of beans. Which of these experimental values has a smaller percent error?
A calculated value of 1342 when the actual value is 1327.
A calculated value of 1327 when the actual value is 1342.
They have the same percent error.
There is not enough information to answer the question.