LESSON 77: What’s in a Mole?: Molar Mass
THINK ABOUT IT
Lead is a highly toxic substance that can be accidentally ingested by humans and animals. Lead atoms interfere with normal processes in the body, causing disturbances in the nervous system. Suppose you have 100 g of lead (II) carbonate, PbCO3, and 100 g of lead (II) chloride, PbCl2. It is the lead atoms specifically that are toxic. If you are exposed to equal masses of lead carbonate and lead chloride, which substance exposes you to more lead atoms and is potentially more toxic? To find out, you need to determine the mass of 1 mol of each.
How can you convert between mass and moles?
To answer this question, you will explore
Molar Mass of Compounds
Comparing a Mole’s Worth
Molar Mass of Compounds
EXPLORING THE TOPIC
Molar Mass of Compounds
COUNTING WITH MOLES
Any object can be counted with units such as a dozen or a million or a mole. Consider a dozen cheese sandwiches. Each sandwich has two slices of bread and one slice of cheese. Therefore, a dozen sandwiches would have a total of two dozen slices of bread and one dozen slices of cheese.
In a similar way, you can count atoms of lead in a lead compound. Because a compound consists of bonded atoms, you can simply add the molar masses of each atom in a molecule or formula unit of the compound to obtain the molar mass of the substance. For example, each formula unit of lead (II) chloride, PbCl2, contains one atom of lead and two atoms of chlorine.
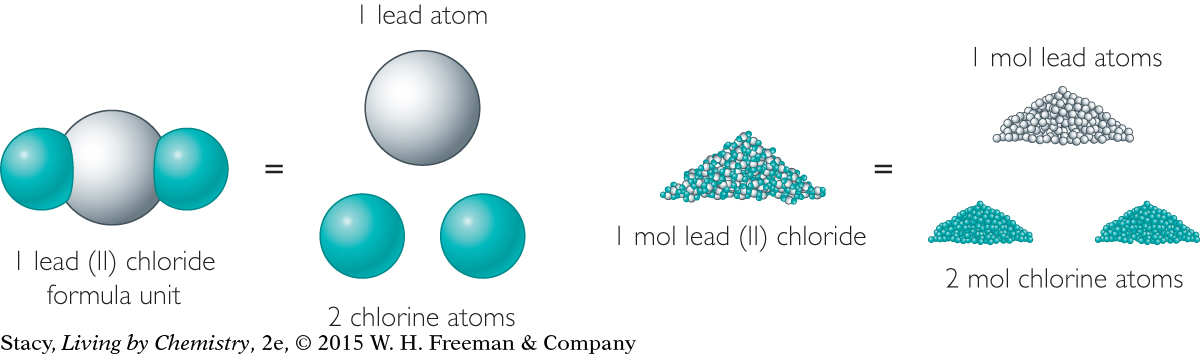
The molar masses of lead and chlorine are 207.2 g/mol and 35.45 g/mol, respectively.
Adding the molar mass of lead and twice the molar mass of chlorine gives the molar mass of lead chloride, PbCl2, which is 278.1 grams per mole, or 278.1 g/mol.
Example 1
The Mass of One Mole
Suppose you have 1 mol of lead, Pb, 1 mol of lead (II) chloride, PbCl2, and 1 mol of lead (II) carbonate, PbCO3. What is the mass of each sample?
Solution
You can find the molar mass of lead, Pb, on the periodic table, 207.2 g/mol.
A mole of PbCl2 has 1 mol of lead atoms and 2 mol of chlorine atoms. You find the molar mass of the compound by adding these molar masses together.
molar mass of PbCl2 = 207.2 + 2(35.45)
= 278.1 g/mol
So the mass of 1 mol of lead (II) chloride is 278.1 g. The molar mass of PbCO3 can be found similarly.
molar mass of PbCO3 = 207.2 + 12.01 + 3(16.00)
= 267.2 g/mol
So the mass of 1 mol of lead (II) carbonate is 267.2 g.
[For a review of this math topic, see MATH Spotlight: Solving Equations on page A-3.]
Comparing a Mole’s Worth
Comparing a Mole’s Worth
HEALTH CONNECTION
HEALTH
CONNECTION
Lead (II) carbonate is one of the white pigments in lead paint. Lead paint dries quickly and forms a durable shiny coating. It is still used to draw lines on pavement, but it is no longer used to paint houses or furniture due to the high toxicity of lead.

A mole of atoms or molecules is usually an amount that you can hold in your hand if the substance is liquid or solid. A table with a “mole’s worth” of a few substances is given below.
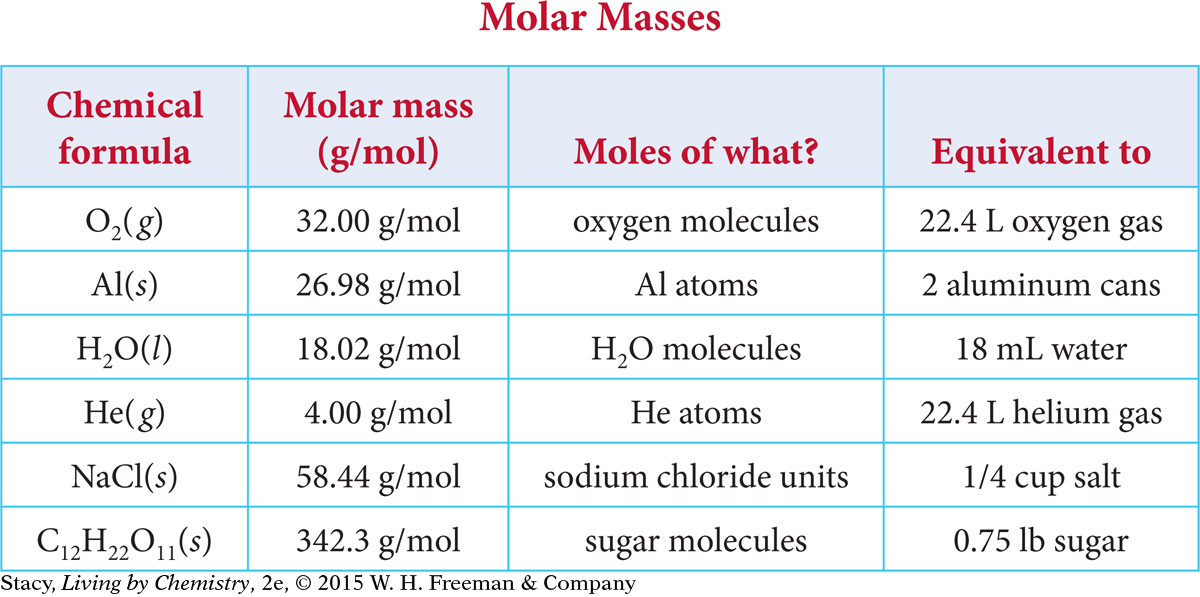
Note that the molar mass of oxygen gas, O2(g), is double the molar mass of oxygen found on the periodic table because oxygen gas is diatomic. Also note that the volume of a mole of O2 gas is the same as the volume of a mole of any other gas at standard temperature and pressure, or STP.
Example 2
Toxicity of Lead Compounds
Which is potentially the most toxic: 1 g lead, Pb, 1 g lead (II) chloride, PbCl2, or 1 g lead (II) carbonate, PbCO3?
Solution
The molar mass of each compound was calculated in Example 1.
molar mass of Pb = 207.2 g/mol
molar mass of PbCl2 = 278.1 g/mol
molar mass of PbCO3 = 267.2 g/mol
Because Pb has the lowest molar mass, 1 g lead, Pb, will have the largest number of moles of lead, so it will potentially be the most toxic, followed by lead (II) carbonate, PbCO3.
LESSON SUMMARY
LESSON SUMMARY
How can you convert between mass and moles?
Chemists compare moles of substances rather than masses of substances because moles are a way of counting atoms, molecules, or units in a compound. The molar mass of a substance is the mass, in grams, of one mole of the substance. The molar mass of a compound is the sum of the molar masses of the atoms in the compound.
Exercises
Reading Questions
Explain how to determine the molar mass of sodium chloride, NaCl.
Describe the approximate size of 1 mol of a solid, a liquid, and a gas. Give a specific example of each.
Reason and Apply
Copy this table and use a periodic table to complete the second column
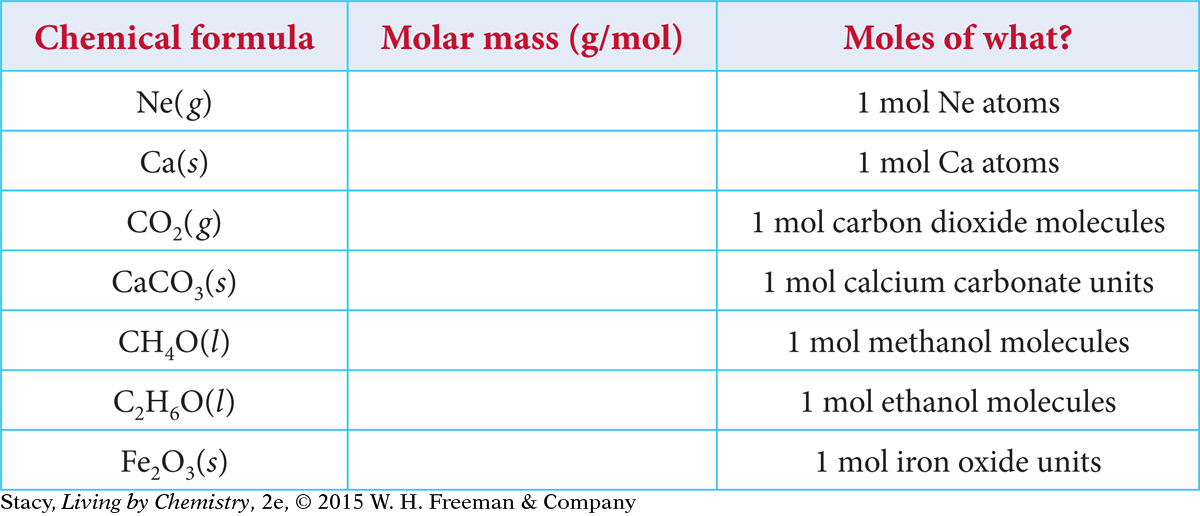 Page 397
Page 397Which has more moles of molecules, 1.0 g methanol, CH4O, or 1.0 g ethanol, C2H6O?
Which has more moles of metal atoms?
10.0 g calcium, Ca, or 10.0 g calcium chloride, CaCl2
5.0 g sodium chloride, NaCl, or 5.0 g sodium fluoride, NaF
2.0 g iron oxide, FeO, or 2.0 g iron sulfide, FeS
How many grams of carbon molecules are in 1 mol of each substance?
methane, CH4
methanol, CH4O
ethanol, C2H6O
What is the mass of 5 mol of iron (III) oxide, Fe2O3?
Which of these has the most chromium?
1.0 g chromium (II) chloride, CrCl2
1.0 g chromium (III) chloride, CrCl3
1.0 g chromium (IV) oxide, CrO2