LESSON 79: How Sweet It Is: Comparing Amounts
THINK ABOUT IT
The safety of artificial sweeteners has been the subject of debate for years. More recently, some schools have banned the sale of both diet and regular soft drinks. Regular soft drinks are sweetened with fructose, while diet soft drinks are usually sweetened with aspartame. If you want to make a healthy decision, you need to consider the toxicities of fructose and aspartame and the amount of each substance in a can of beverage.
How can you use moles to compare toxicity?
To answer this question, you will explore
Comparing Moles of Sweeteners
Toxicity of Sweeteners
Comparing Moles of Sweeteners
EXPLORING THE TOPIC
Comparing Moles of Sweeteners
CONSUMER CONNECTION
CONSUMER
CONNECTION
The compounds aspartame, saccharin, and sucralose are the most common artifical sweeteners in use today. Aspartame is the sweetener most commonly used in diet soft drinks. Saccharin is often used as a sweetener in toothpastes.

It is hard for many of us to imagine a day without sugar. From cereal to flavored drinks and candy, our intake of sugar adds up. So, when the first artificial sweeteners came onto the market in the 1950s, it seemed a good idea to use this low-calorie substitute for a favorite ingredient.
Two sweeteners, fructose, C6H12O6, and aspartame, C14H18N2O5, are shown. Notice that they are both medium-sized molecules made up of carbon, hydrogen, and oxygen atoms. The artificial sweetener, aspartame, also has two nitrogen atoms in its molecules.
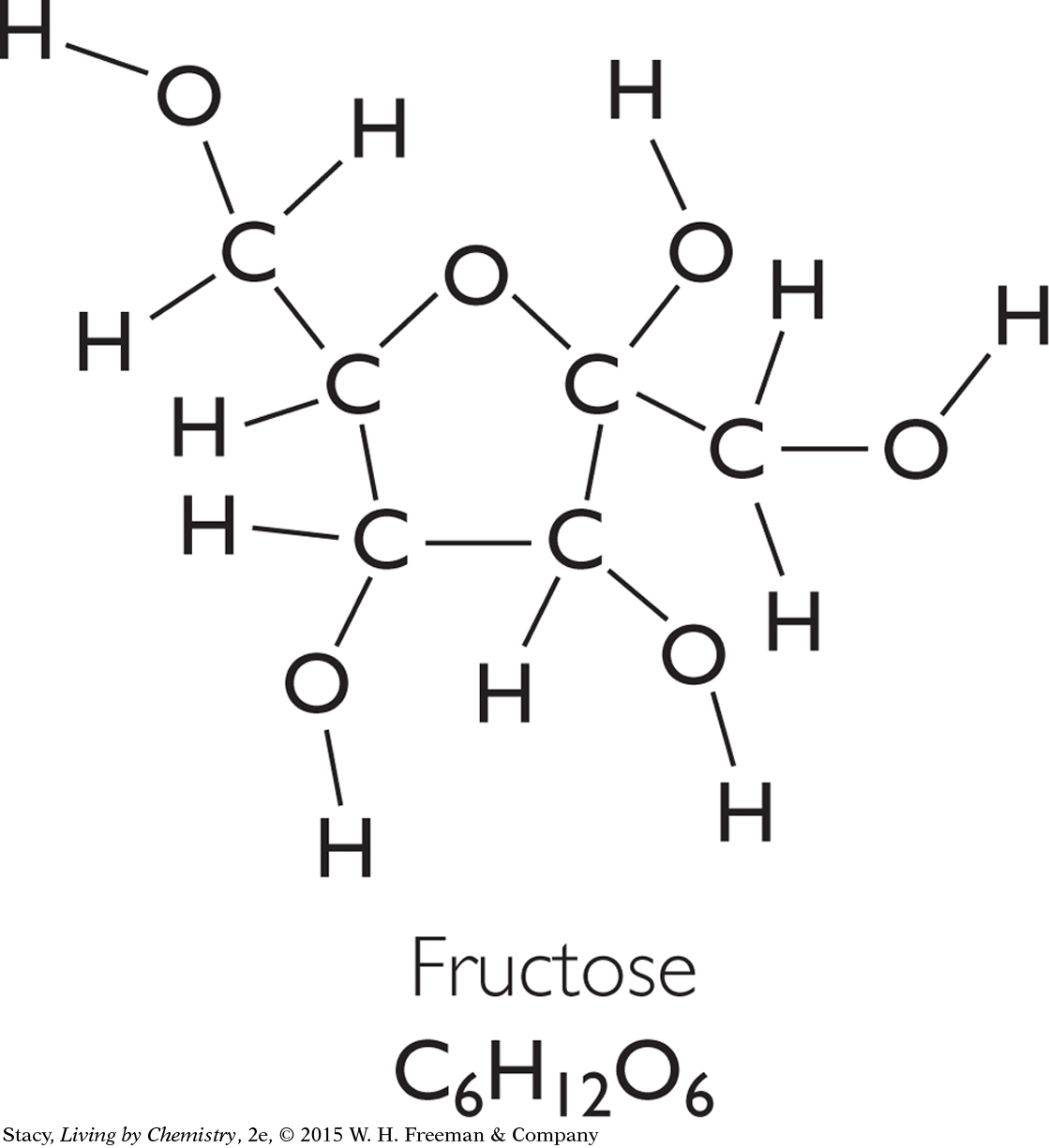
Molar mass: 180.1 g/mol
|
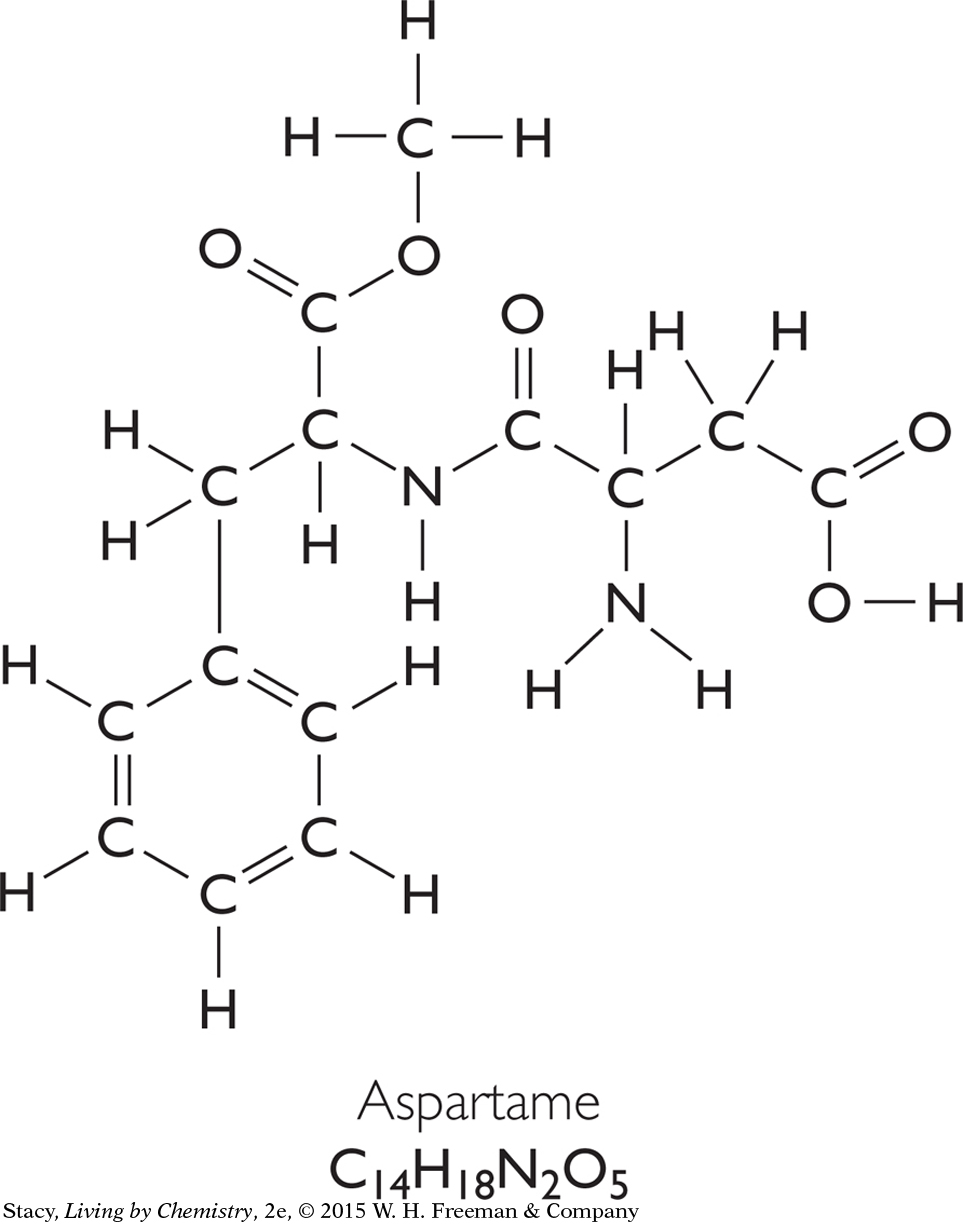
Molar mass: 294.3 g/mol
|
The molar masses of fructose and aspartame represent the mass per mole of molecules. Recall that you can obtain the molar mass of a molecule by adding the molar masses of each atom in the molecule.

The molar mass of fructose is less than that of aspartame. A 1 g sample of fructose will contain more molecules than a 1 g sample of aspartame because each fructose molecule has a smaller mass than an aspartame molecule.
A can of regular soft drink has about 40.0 g of fructose as a sweetener. A can of diet soft drink has about 0.225 g of aspartame as a sweetener. You can determine the number of molecules of sweetener in each can using the molar mass of each substance.
| Fructose = (regular soft drink) | Aspartame = (diet soft drink) |
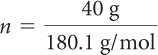
|
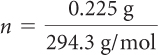
|
| = 0.22 mol fructose | = 0.00076 mol aspartame |
A diet soft drink needs only 0.00076 mol of aspartame molecules to replace the sweetness of 0.22 mol of fructose molecules in a regular soft drink. So you need significantly less aspartame to obtain the same sweetness provided by fructose.
Toxicity of Sweeteners
Toxicity of Sweeteners
HEALTH CONNECTION
HEALTH
CONNECTION
When aspartame is broken down in the body, it produces methanol and two amino acids: aspartic acid and phenylalanine. People with a rare genetic disorder called phenylketonuria can’t metabolize phenylalanine and experience toxic effects. For this reason, a warning label about phenylalanine must be placed on all products containing aspartame.

Artificial sweeteners allow people with diabetes, and others who must avoid sugar, to enjoy foods that would otherwise be dangerous for them to eat. Artificial sweeteners do not cause cavities, and because they are not digested, artificial sweeteners do not provide extra calories to the body. However, the safety of artificial sweeteners continues to be a subject of debate.
To make an informed decision about whether you want to consume artificial sweeteners, it is useful to compare the toxicities of fructose and aspartame. Take a moment to review this information about each sweetener.
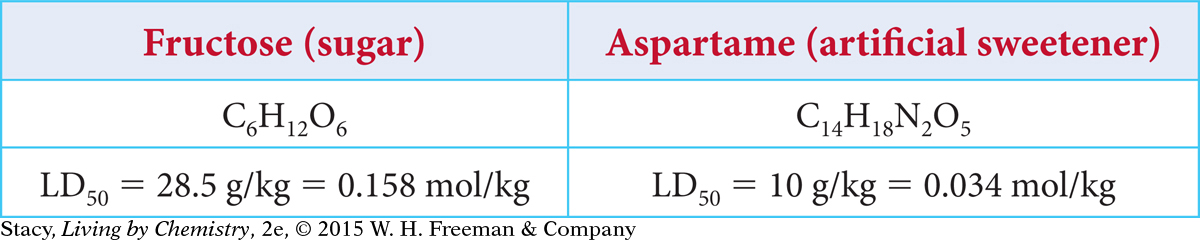
The LD50 of fructose in grams is about three times larger than the LD50 for aspartame (30 g per kg versus 10 g per kg). The LD50 in moles is almost five times larger for fructose than for aspartame (0.158 mol per kg versus 0.034 mol per kg). So, the sweetner aspartame is more toxic than fructose if you consume similar amounts of each.
Example
How Many Cans?
How many cans of a regular soft drink does a 64 kg (141 lb) person have to drink in one sitting to exceed the lethal dose of fructose? How many cans of diet soft drink does the same person have to drink in a short time to exceed the lethal dose of aspartame?
Solution
First, determine how many grams of each sweetener are lethal for a 64 kg person. Then, take this number of grams and divide by the grams of each sweetener in a can of soft drink to get the number of cans of soft drink that would be lethal.
| Regular soft drink | Diet soft drink | |
| Determine the lethal dose for each sweetener using LD50 values. | 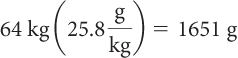
|
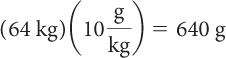
|
| Divide by the mass of sweetener in one can to get number of cans. | 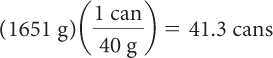
|
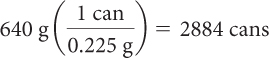
|
It would take about 41 cans of regular soft drink to reach the lethal dose of fructose and 2884 cans of diet soft drink to reach the lethal dose of aspartame.
HEALTH CONNECTION
HEALTH
CONNECTION
In 1958, Congress passed the Delaney Clause of the Food, Drug, and Cosmetic Act. The clause prohibited the use of all food additives found to be carcinogenic. As a result, several artificial sweeteners have been banned by the Food and Drug Administration over the years.
It would be difficult to drink enough regular soda or diet soda to reach the lethal dose in a short period of time. So it is highly unlikely that exposure to either fructose or aspartame would be lethal in the short term. However, it is important to note that the LD50 is not a good measure of health effects from long-term exposure. It is probably best to limit intake of both sugar and aspartame.
LESSON SUMMARY
LESSON SUMMARY
How can you use moles to compare toxicity?
Chemists often find it useful to compare moles of substances rather than masses of substances. Moles allow you to compare numbers of molecules or formula units in your sample. Substances with large molar masses contain fewer molecules per gram than substances with smaller molar masses. The health effect of exposure to a toxic substance depends on both the LD50 and the amount of the substance.
Exercises
Reading Questions
What evidence shows that aspartame is sweeter than fructose?
What evidence shows that it would be difficult to exceed the lethal dose of aspartame?
Reason and Apply
There are 25 mg of caffeine, C8H10N4O2, in a can of regular soft drink. The LD50 for caffeine, C8H10N4O2, is 140 mg/kg.
How many cans of regular soft drink can a 65 kg person drink in a short period of time before exceeding the lethal dose?
What is the toxicity of caffeine in moles per kilogram?
The LD50 for saccharin, C7H5NO3S, is 14.2 g/kg. If you have 1 mol of aspartame and 1 mol of saccharin, which would be more toxic? Show your work.
Write an argument for or against the use of artificial sweeteners. Be sure to provide evidence to support your argument. Cite at least two references that you use.