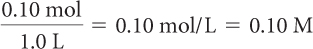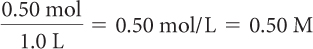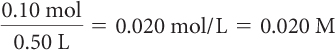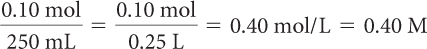LESSON 80: Bearly Alive: Solution Concentration
409
THINK ABOUT IT
When a toxic substance is in solid form, keeping track of toxic amounts is fairly simple. But what happens if the toxic substance is dissolved in water? For example, the water that comes out of your tap is probably a solution containing small amounts of dissolved chlorine. Small amounts of chlorine benefit the water supply by killing bacteria, but large amounts would be toxic to people who drink the water. It is important to have methods for tracking substances dissolved in water.
How can you keep track of compounds when they are in solution?
To answer this question, you will explore
Solutions
Solution Concentration
Solutions
EXPLORING THE TOPIC
Solutions

A solution is a mixture of two or more substances that is uniform throughout. This means it is uniform at the molecular, ionic, or atomic level. The ocean, a soft drink, liquid detergent, window cleaner, and even blood plasma are all examples of solutions. A glass of powdered drink has many dissolved substances in it, but they are mixed so well that the individual substances in the solution are no longer distinguishable.
Each solution listed above consists of one or more substances dissolved in water. The water in these solutions is called the solvent. There are many other solvents besides water, such as alcohol, turpentine, and acetone. So the solvent is the substance that other substances are dissolved in. A solvent does not necessarily have to be a liquid. Solvents can be gases, liquids, or solids. Water is one of the most important solvents on Earth because it is so common in living systems.
A substance that is dissolved in a solvent is called the solute. Solutes can be gases, liquids, or solids. In a sugar solution, the sugar is the solute and water is the solvent. In a carbonated soft drink, carbon dioxide gas is one of the solutes dissolved in the soft drink.
410

A solution is considered a homogeneous mixture because of its uniformity. Mixtures that are not uniform throughout are called heterogeneous. Salad dressing or mixed nuts are examples of heterogeneous mixtures.
Solution Concentration
Solution Concentration
The concentration of a solution refers to the amount of a substance dissolved in a specified amount of solution. The concentration of a solution depends on both the quantity of solute and the quantity of solvent. There are several ways of measuring these quantities. A common way to measure concentration is to determine the number of moles of solute per total volume of solution in liters. This is called the molarity of a solution and is expressed in moles per liter, mol/L. Notice that solution concentration is the same as number density, n/V.
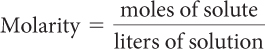
Consider the two solutions in the illustration. Both contain a blue dye. Dyes are generally molecular substances that make a solution appear to be a certain color. The concentration of blue dye is greater in the first solution even though the volume of solution is smaller. You can tell this because the solution on the left is darker than the solution on the right.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Water-soluble dyes are used in preparing a lot of our foods. One form of blue dye that goes into our food is indigo, C16H10N2O2. Indigo is used to dye denim cloth for blue jeans. When this dye is put in food products, it is referred to as FD&C Blue No. 2.

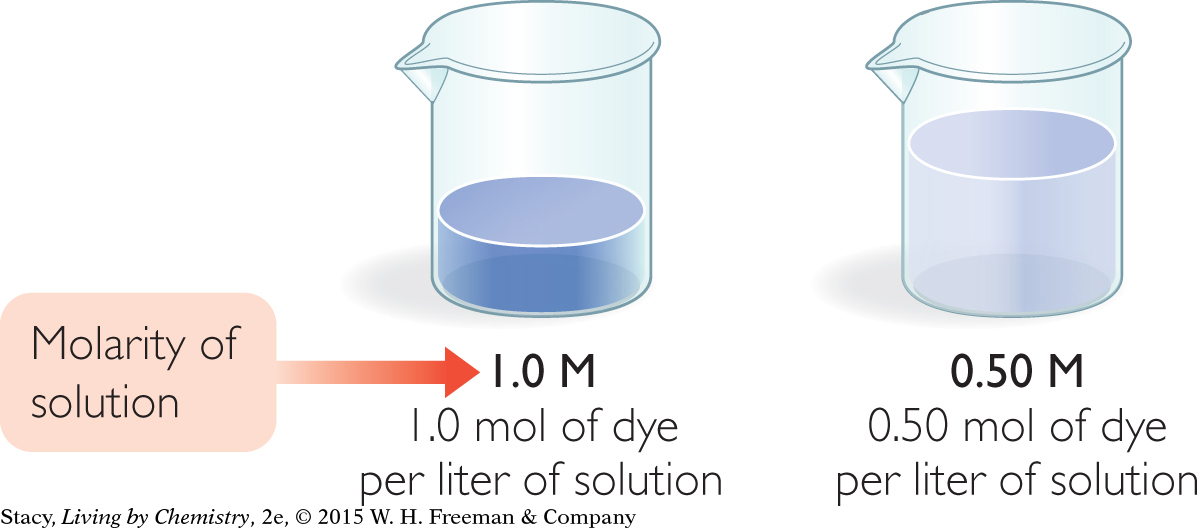
The M is an abbreviation for molar, which means moles of solute per liter of solution. The dye solution on the left is 1.0 molar, 1.0 M, while the one on the right is 0.50 molar, or 0.50 M.
If you want to make a solution more concentrated, you need to add solute. If you want to make a solution more dilute, or less concentrated, you need to add water.
Example 1
Molarity
Determine the molarity of each.
0.10 mol of NaCl in 1.0 L of solution
0.50 mol of NaCl in 1.0 L of solution
0.10 mol of NaCl in 0.50 L of solution
0.10 mol of NaCl in 250 mL of solution
Solution
Molarity is the number of moles of solute divided by the liters of solution.
411
CALCULATING MOLARITY FROM MASS IN GRAMS
In the classroom, you were challenged to create a saturated sugar solution and a saturated salt solution. A saturated solution is one that contains the maximum amount of dissolved solute possible in a certain volume of liquid. To make a saturated solution, you must add solid and stir until no more solid dissolves. You can calculate how many grams of each solid you added if you find the mass of the solutions before and after you added the solute. The molar mass of a substance allows you to convert the mass of solute in grams to moles of solute. You can then determine the molarity of each solution.
Big Idea
Big Idea
Molarity keeps track of the number of dissolved particles in a liquid.
Example 2
Finding Molarity
What is the concentration in moles per liter of a 250 mL solution that contains 16.0 g of dissolved sugar, C12H22O11?
Solution
First, convert the mass of sugar to moles by dividing by the molar mass of sugar, C12H22O11. Next, to determine molarity, divide the number of moles by the volume.
| Convert mass to moles. | 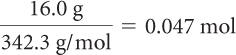
|
| Calculate the molarity. | 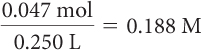
|
412
LESSON SUMMARY
LESSON SUMMARY
How can you keep track of compounds when they are in solution?
KEY TERMS
solution
solvent
solute
homogeneous mixture
heterogeneous mixture
concentration
molarity
molar
saturated solution
A solution is a mixture of two or more substances that is uniform throughout. To track substances that are in solution, chemists measure the number of particles per liter of liquid. This is called the concentration or the molarity of the solution. Molarity is a measure of number density and is expressed in units of moles per liter of solution.
Exercises
Reading Questions
What does it mean that a solution is “uniform throughout”?
What is the difference between the concentration of a solution and the volume of a solution?
Reason and Apply
Find at least three solutions at home. Identify the solute and the solvent for each.
Find at least three mixtures at home that are not solutions. Identify the substances in each mixture.
Place these salt solutions, NaCl(aq), in order of increasing molarity.
4.0 mol per 8.0 L
6.0 mol per 6.0 L
1.0 mol per 10 L
Determine the molarity for each of these salt solutions, NaCl(aq). Then list the solutions in order of increasing molarity.
29.2 g per 0.50 L
5.8 g per 50 mL
2.9 g in 10.2 mL
Determine the molarity for each of these solutions. Then list the solutions in order of increasing molarity.
25 g C6H12O6 per 0.25 L
50 g C6H12O6 per 0.25 L
50 g C12H22O11 per 0.25 L
Which of the substances listed here represent a heterogeneous mixture? Choose all that apply.
the air in your classroom
beef stew
mouthwash
chocolate pudding
How can you increase the molarity of a solution?
Add solute.
Add solvent.
Pour out some of the solution.
All of the above.
When expressing molarity, M stands for
atoms per liter of water.
moles per milliliter of solvent.
moles per liter of solution.
atoms per milliliter of solute.
grams per liter of solution.