LESSON 82: Holey Moley: Preparing Solutions
THINK ABOUT IT
CONSUMER CONNECTION
CONSUMER
CONNECTION
Water that has a high mineral content is called hard water, while water with a low mineral content is called soft water. Soap and shampoo do not lather up well in very hard water and don’t rinse out easily in very soft water. Minerals commonly found in tap water are calcium and magnesium carbonates, and they sometimes build up as deposits in pipes and on faucets.

Human blood plasma contains a certain molarity of dissolved salt, NaCl. If you are taken to the hospital in an ambulance and given an intravenous, or IV, solution, it is critical that the salt concentration match that of your blood, about 0.15 M NaCl. Otherwise, the IV solution could be toxic to you.
How can you create a solution with a specific molarity?
To answer this question, you will explore
Creating Solutions
Relating Mass, Moles, and Volume
Creating Solutions
EXPLORING THE TOPIC
Creating Solutions
Suppose a lab technician wants to make a 0.15 M sodium chloride solution. How should this solution be prepared?
PREPARING A 0.15 M SALT SOLUTION
A concentration of 0.15 M means that 0.15 mol of salt is dissolved in 1 L of solution. Therefore, if you want to make 1 L of solution, you will need to measure out 0.15 mol of solid sodium chloride, NaCl.
To know how much salt to weigh out, you must convert moles to mass using the molar mass of the compound. In this case, simply add the molar masses for Na and Cl from the periodic table. The molar mass of NaCl is 58.4 g/mol to one decimal place. Now multiply the moles of NaCl you want by the molar mass to get the number of grams of NaCl you need for your solution.
Mass = molar mass · n = (58.4 g/mol)(0.15 mol) = 8.76 g NaCl
If you dissolve 8.76 g NaCl in water so that the total volume of solution is 1 L, you will have a solution that is 0.15 M, or 0.15 mol/L NaCl.
Important to Know
When you dissolve one substance in another, they both take up space. So, to make 1 L of an aqueous solution, you add less than 1 L of water.
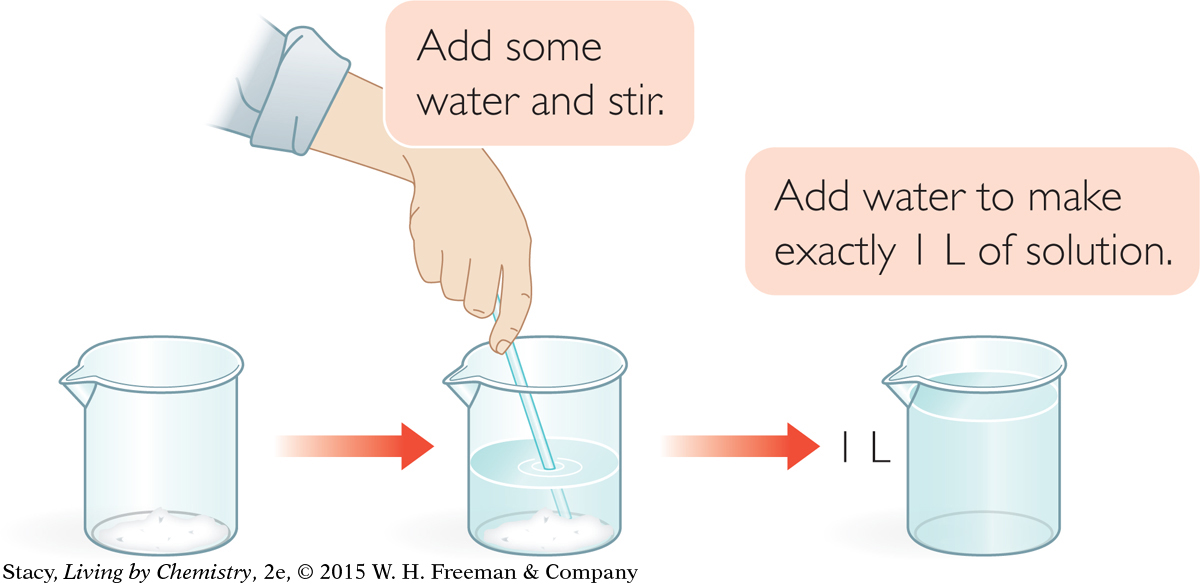
Example 1
Dextrose Solution
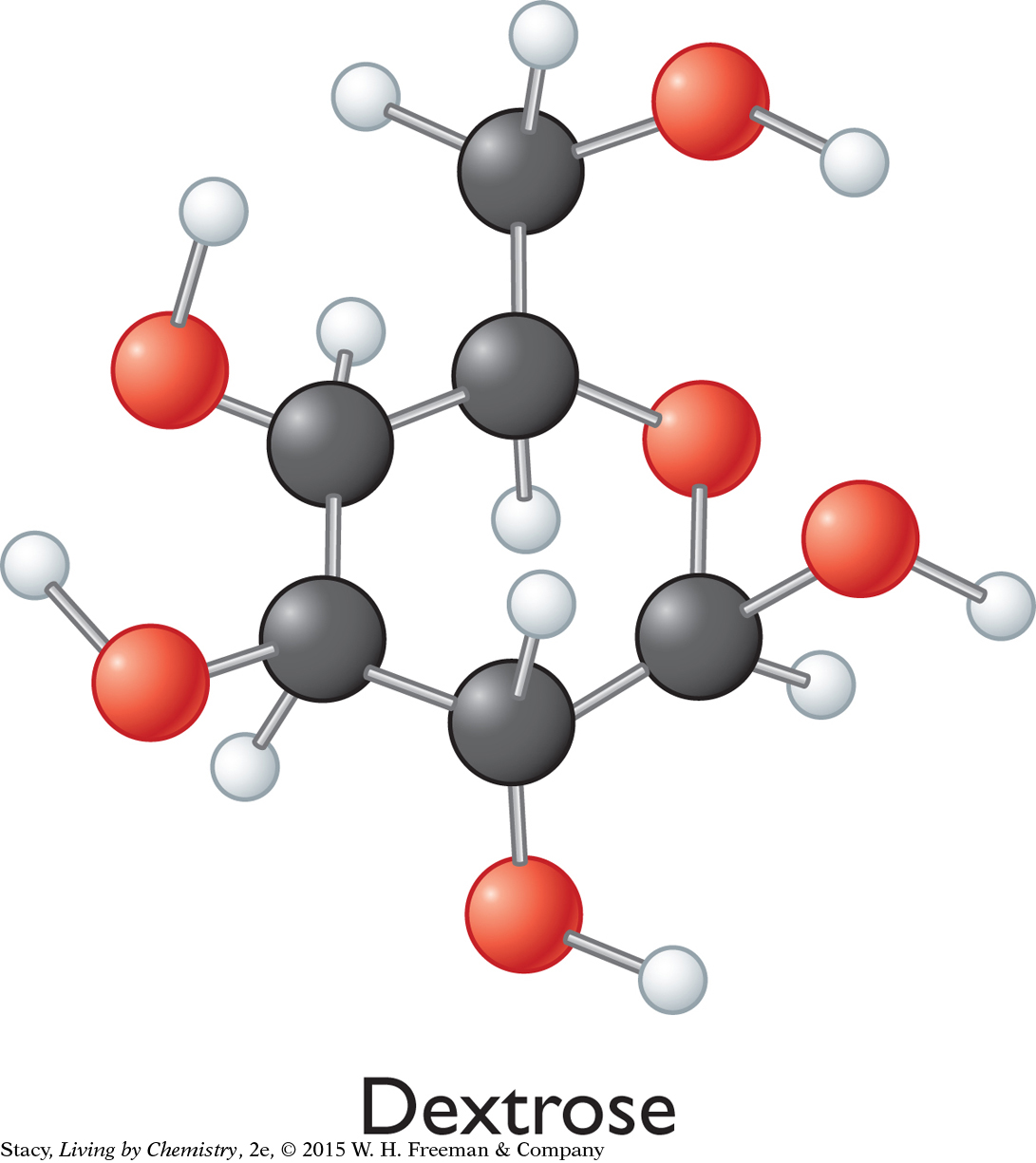
Sometimes doctors administer an IV solution containing a solution of dextrose to a patient who has low sugar or high sodium in the bloodstream. Dextrose is a type of sugar. The structural formula for dextrose is shown in the illustration.
The IV solution contains 150 g of dextrose in 3 L of solution. What is the molarity of this solution?
Solution
You need to convert grams of dextrose to moles of dextrose to determine the molarity. You know there are 150 g of dextrose in 3 L, or 50 g/L.
First, determine the molar mass of dextrose. As you can see from the ball-and-stick model, there are 6 C atoms, 12 H atoms, and 6 O atoms, so the formula is C6H12O6.
| Find the molar mass of dextrose, C6H12O6. | 180.2 g/mol |
| Divide grams of solute by the molar mass to obtain moles. | 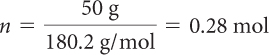
|
There is 0.28 mol of dextrose in 1 L of solution, so the molarity is 0.28 M.
Relating Mass, Moles, and Volume
Relating Mass, Moles, and Volume
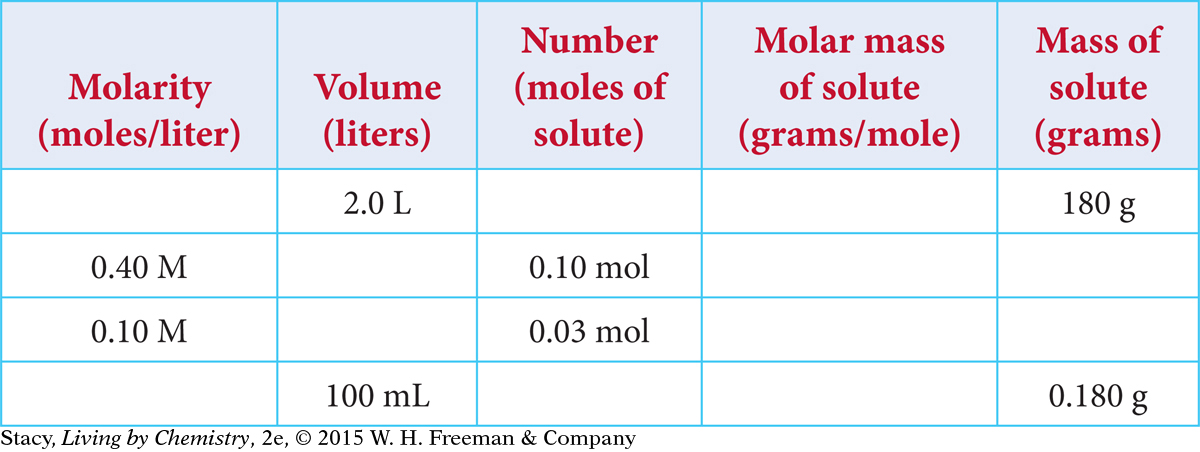
Just as it is important to convert mass to moles, it is also important to be able to convert moles to mass. The table below shows data for four samples of sucrose, C12H22O11, each with a different volume and molarity. How many grams of sucrose are dissolved in each sample?
To find the moles of solute in each sample, multiply the values in the second and third columns. To find the mass of solute in each sample, multiply the fourth and fifth columns.
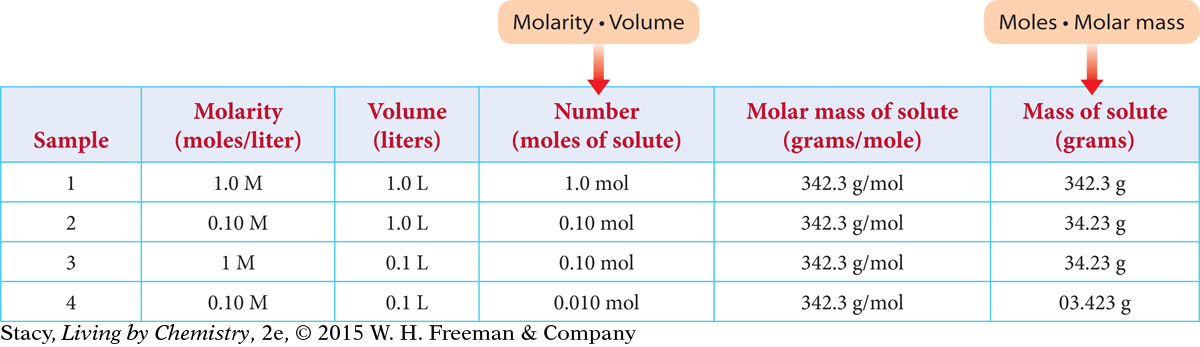
Notice that there is more than one way to make a 0.10 M solution. For example, you can dissolve 0.10 mol to make 1.0 L or you can dissolve 0.010 mol to make 100 mL (0.10 L).
Example 2
Guidelines for Safe Drinking Water
Arsenic, As, is an element that is found naturally in the soil. As a result, tiny levels of arsenic are often found in some water supplies. Like that of all substances, the toxic effect of arsenic is dependent on the dosage you receive. For this reason, the Environmental Protection Agency, or EPA, sets limits on the amounts of dissolved substances that can be present in our drinking water. The EPA has set the upper limit for arsenic in drinking water at 0.00010 g/L.
Is it safe to drink water that has a concentration of 0.000020 M As?
Solution
The EPA limit is given in grams/liter, and the concentration of the solution is in moles/liter. So you need to convert moles to grams (or vice versa) to make a comparison.
The proportionality constant between grams of arsenic and moles of arsenic is the molar mass, which you can find on the periodic table. To find the mass of arsenic, multiply the molar mass by the number of moles.
| Look up the molar mass of As. | 74.9 g/mol |
| Mass = molar mass · n | (74.9 g/mol)(0.000020 mol) = 0.0015 g As |
The 0.000020 M arsenic solution has 0.0015 g of As per liter of solution. This amount is 15 times the concentration allowed by the EPA. So, the solution is not safe for drinking.
LESSON SUMMARY
LESSON SUMMARY
How can you create a solution with a specific molarity?
Molar mass values on the periodic table allow you to convert between grams and moles. The amount in grams of solid needed to create solutions of specific molarities can be calculated using the molar mass of the solid. Likewise, the mass of solid dissolved in a solution can be determined if the concentration and volume of the solution are known.
Exercises
Reading Questions
Explain how you would prepare a solution of sucrose with a molarity of 0.25.
How are mass of solute, moles of solute, and volume of solution related?
Reason and Apply
How many grams of solute do you need to make 1 L of each of the solutions listed?
0.50 M NaCl
2.0 M CaCl2
1.5 M NaOH
Page 421Copy this table and complete it for solutions of glucose, C6H12O6. Remember to convert milliliters to liters.
What volume of each of these solutions would contain 58.44 g of NaCl?
0.10 M NaCl
3.0 M NaCl
5.5 M NaCl
How many grams of fructose, C6H12O6, are in 1 L of soft drink if the molarity of fructose in the soft drink is 0.75 M?
Which is more concentrated: a 1.0 L solution with 20 g of sucrose, C12H22O11, or a 1.0 L solution with 20 g of glucose, C6H12O6?
The Environmental Protection Agency has set the upper limit for fluorine, F, in drinking water at 0.0040 g/L of water. Is it safe to drink a glass of water in which the concentration of fluorine is 0.00010 M?