LESSON 85: Pass the Proton: Acid and Base Theories
THINK ABOUT IT
People have known for thousands of years that vinegar, lemon juice, green apples, and many other foods taste sour. However, it is only recently that scientists have presented theories to explain what all these substances have in common. In what ways are these sour-tasting substances similar, and why are they defined as acids?
How are acids and bases defined?
To answer this question, you will explore
Chemical Makeup of Acids and Bases
Acid and Base Theories
Strong and Weak Acids and Bases
Chemical Makeup of Acids and Bases
EXPLORING THE TOPIC
Chemical Makeup of Acids and Bases
Many substances dissolve in water to produce solutions that are acidic. Because the behavior of all acidic solutions is similar, you might expect some similarity in the chemical makeup of these substances.
Take a moment to look for patterns in the table of common acids.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The human stomach secretes hydrochloric acid, HCl, which begins the digestion process for large protein molecules and also provides a barrier against bacteria, fungi, and other microorganisms naturally found in food and water. Stomach acid is also necessary for the proper absorption of certain minerals such as iron and zinc as well as some of the B vitamins.

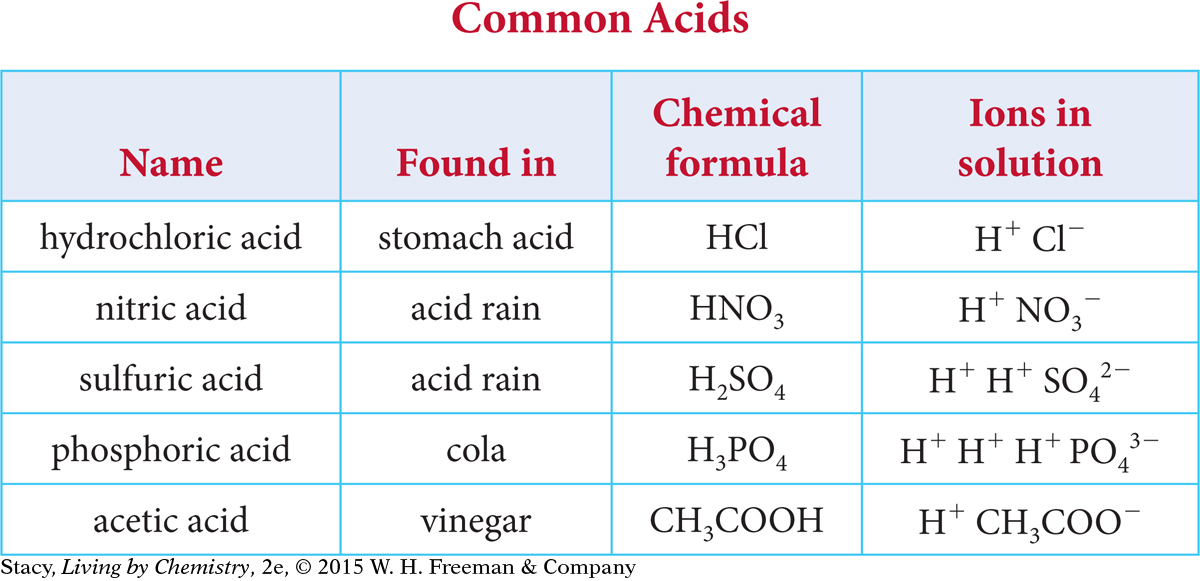
Here are some patterns you might notice.
The acids are made of main group nonmetal atoms, such as carbon, oxygen, and chlorine, bonded covalently.
The only element that they all have in common is hydrogen.
They all break apart, or dissociate, in solution to form at least one hydrogen cation, H+, and an anion.
You might also notice that some of the molecular formulas are written in a way that gives some information about the molecular structure. For example, acetic acid, C2H4O2, is written as CH3COOH.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Lye, or sodium hydroxide, is a corrosive substance found in many household items including oven cleaner and drain opener. Lye is also used for curing foods such as green olives and century eggs and for glazing pretzels. It is also used in soap making.

Many other substances dissolve in water to produce solutions that are basic. If acidic solutions have H+ ions in common, what is similar in the chemical makeup of basic solutions?
Take a moment to look for patterns in the names and chemical formulas of the common bases listed in this table.
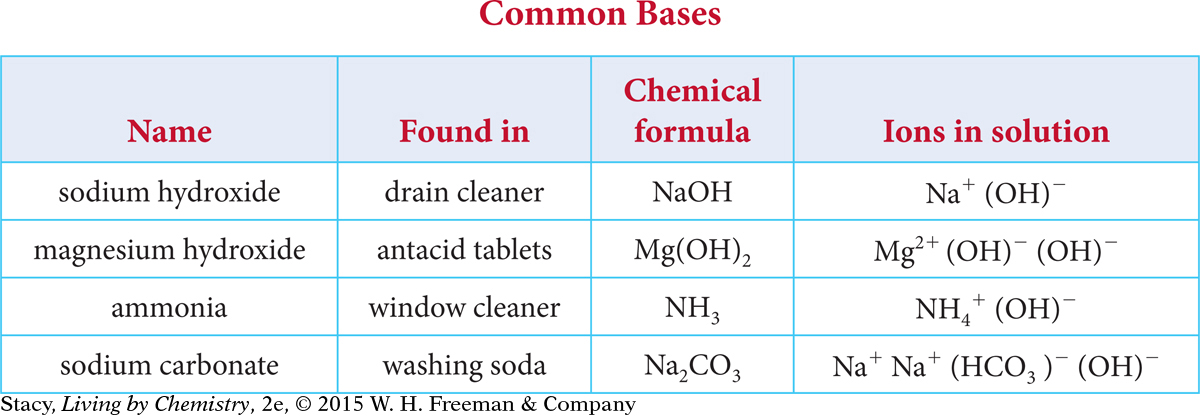
Here are some things you might notice.
Many of the bases contain a metal atom and a hydroxide ion, OH−.
The metal atoms are in the first two columns of the periodic table.
Some compounds, such as ammonia and sodium carbonate, produce OH− when dissolved in water, even though there is no OH− in the chemical formula.
Except for the two hydroxides, their names do not reveal that they are bases.
Acid and Base Theories
Acid and Base Theories
ARRHENIUS THEORY OF ACIDS AND BASES
One of the early theories of acids and bases was first proposed in 1884 by a Swedish chemist named Svante Arrhenius. He defined an acid as a substance that adds hydrogen ions, H+, to an aqueous solution. He defined a base as a substance that adds hydroxide ions, OH−, to an aqueous solution.
BRØNSTED-LOWRY THEORY OF ACIDS AND BASES
The Arrhenius definitions of acids and bases do not explain how substances without hydroxide ions, OH−, in their formula, like ammonia, NH3, can be bases. In the early 1920s, a Danish chemist, Johannes Brønsted, and an English chemist, Thomas Lowry, proposed a slightly different definition for acids and bases to account for this chemical behavior. They defined an acid as a substance that can donate a proton to another substance. They defined a base as a substance that can accept a proton from another substance. A proton in this case is actually a hydrogen ion, H+. Because a hydrogen atom typically has no neutrons, if you remove the electron from a hydrogen atom to form an ion, all that’s left is a proton.
When ammonia, NH3, is added to water, it removes a hydrogen ion, H+, from some of the water molecules, leaving behind some hydroxide ions, OH−. An NH3 molecule accepts a hydrogen ion and becomes NH4+. This makes NH3 a Brønsted-Lowry base.
NH3(g) + H2O(l) → NH4+(aq) + OH−(aq)
So dissolved ammonia really consists of ammonium and hydroxide ions. For this reason, it is often referred to as ammonium hydroxide solution. Many substances that do not contain OH− can act as bases.
Strong and Weak Acids and Bases
Strong and Weak Acids and Bases
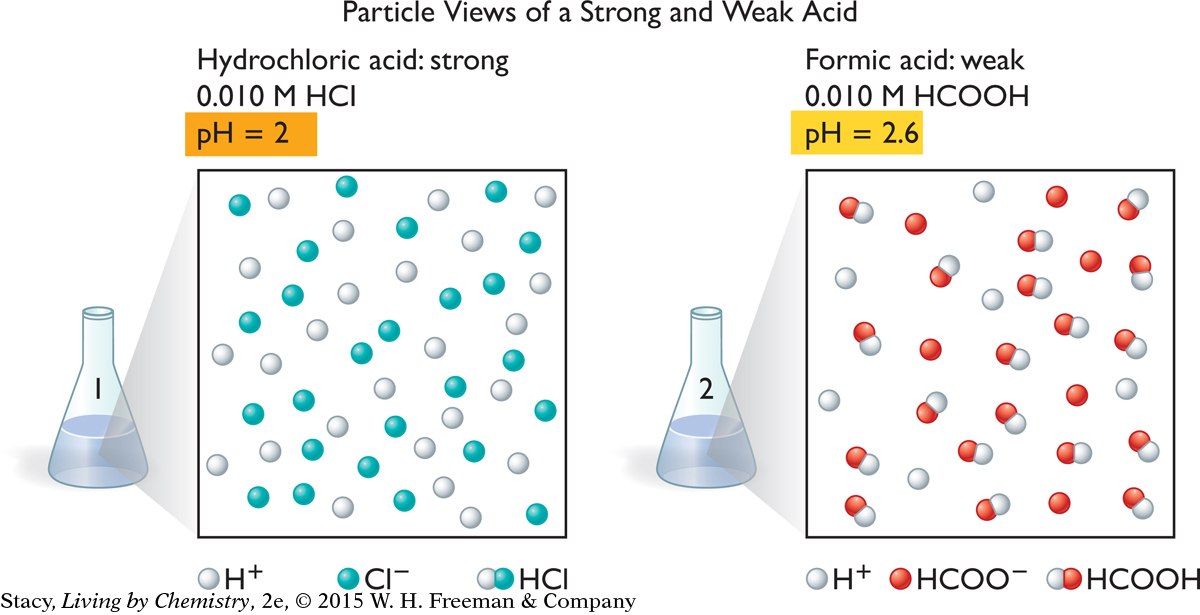
These two illustrations show particle views of a 0.010 M hydrochloric acid, HCl, solution and a 0.010 M formic acid, HCOOH, solution. The water molecules are not shown. Take a moment to examine them.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Formic acid, HCOOH, is the simplest carboxylic acid. It is most famous for its presence in bee stings and ant bites. In fact, the word formic comes from formica, the Latin word for ant.

Notice that there are no molecules of HCl in the solution on the left. The HCl has dissociated completely into H+ and Cl− ions. However, the solution on the right contains formic acid molecules. Only some of the HCOOH molecules have dissociated into ions. This means that the concentration of H+ ions is smaller in the formic acid solution than in the hydrochloric acid solution, even though the solutions have the same molarities.
Acids that dissociate completely in solution are called strong acids. Strong acids include hydrochloric acid, HCl, nitric acid, HNO3, sulfuric acid, H2SO4, and hydrobromic acid, HBr. Strong acids are good conductors of electricity.
Acids that dissociate only partially in solution are called weak acids. These solutions are only moderate conductors of electricity. Some common weak acids are formic acid, HCOOH, acetic acid (vinegar), CH3COOH, citric acid, C3H5O(COOH)3, and phosphoric acid, H3PO4. Weak acids tend to be less corrosive because they do not dissociate completely into ions.
Bases can also be classified as strong or weak. A strong base dissociates completely into ions in solution and weak bases dissociate only partially. Some examples of strong bases include sodium hydroxide, NaOH, and barium hydroxide, Ba(OH)2. Examples of weak bases include ammonia, NH3, and aniline, C6H5NH2.
Big Idea
Big Idea
Strong acids and strong bases dissociate completely into ions. Weak acids and weak bases dissociate only partially into ions.
LESSON SUMMARY
LESSON SUMMARY
How are acids and bases defined?
KEY TERMS
dissociate
strong acid
weak acid
strong base
weak base
The Arrhenius definition of an acid is a compound that dissociates in solution to form a hydrogen ion, H+, and an anion. The Arrhenius definition of a base is a substance that dissociates in solution to form a hydroxide ion, OH−, and a cation. However, not all bases contain OH−. Brønsted and Lowry defined acids as proton donors and bases as proton acceptors. The word “proton” in this case refers to an H+ ion. A strong acid or base dissociates completely into ions in solution. A weak acid or base dissociates only partially into ions in solution.
Exercises
Reading Questions
According to the Arrhenius theory, what is an acid and what is a base?
How is the Brønsted-Lowry theory of acids similar to the Arrhenius theory, and how is it different?
What is the difference between a strong acid and a weak acid? Draw a picture showing the particle view of each.
Reason and Apply
Label the substances listed below as acids or bases. In each case, list the ions you would expect to find in solution.
hydroiodic acid, HI
formic acid, HCOOH
rubidium hydroxide, RbOH
hypochlorous acid, HOCl
selenous acid, H2SeO4
phosphine, PH3
perchloric acid, HClO4
calcium hydroxide, Ca(OH)2
Consider a solution of hydrobromic acid, HBr.
If you draw a particle view of this acid with 10 H+ ions, how many Br− ions would you need? Explain your thinking.
Sketch a particle view of an HBr solution with 10 H+ ions.
Consider a solution of magnesium hydroxide, Mg(OH)2.
If you draw a particle view of this base with 10 Mg2+ ions, how many OH− ions would you need? Explain your thinking.
Sketch a particle view of a Mg(OH)2 solution with 10 Mg2+ ions.
Explain why aqueous washing soda, Na2CO3, is a basic solution.
A solution of 0.10 M hydrochloric acid, HCl, is a better conductor of electricity than 0.10 M acetic acid, CH3COOH. Sketch the ions and molecules in both solutions to explain this observation.