Featured LAB: Neutralization Reactions
Featured LAB
Neutralization Reactions
!
SAFETY
Instructions
Safety goggles must be worn at all times.
Acids and bases are corrosive. Do not get any on skin or near eyes.
In case of a spill, rinse with large amounts of water.
Purpose
To examine reactions between acids and bases.
Procedure
Add 20 drops of each solution to the well plate as specified in the illustration.
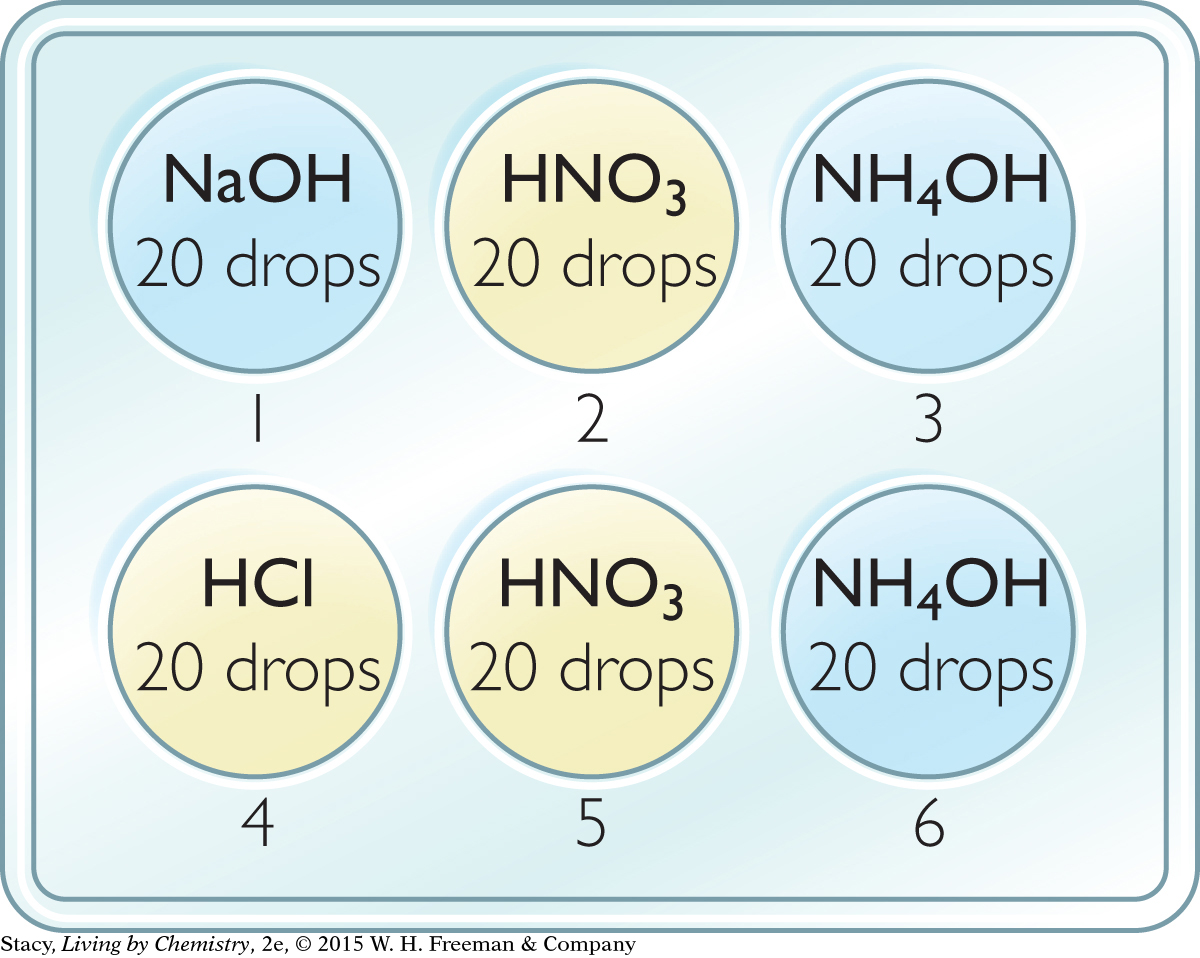
Add 1 drop of bromothymol blue indicator to each well. Bromothymol blue is yellow in acid, blue in base, and green in neutral solution.
Test with HCl: Try to turn the solutions in wells 1, 2, and 3 green using drops of 0.10 M HCl. Record your observations in a data table.

Materials
well plate
set of five labeled dropper bottles: 0.10 M HCl (hydrochloric acid), 0.10 M HNO3 (nitric acid), 0.10 M NaOH (sodium hydroxide), 0.10 M NH4OH (ammonium hydroxide), and bromothymol blue indicator

Test with NaOH: Try to turn the solution in wells 4, 5, and 6 green using drops of 0.10 M NaOH.
Analysis
What did you observe when you mixed an acid with a base?
Acids react with bases to form an ionic salt and water. Copy this chemical equation and label the acid, base, and ionic salt.
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
Complete and balance the equation for the reaction in each well. If no reaction occurred, simply write “no reaction” on the products side of the equation.
What combinations of reactants do not yield new products?
Name the three salts produced in the reactions.
Making Sense List three things you learned as a result of performing this lab procedure.