LESSON 96: Point of View
THINK ABOUT IT
Suppose that you have just come inside on a very cold day. There is a fire in the fireplace. If you go near the fire, you will feel the warmth. Heat is transferred to your body by the warm gases of the flames and the energy radiating from the burning wood.
What do temperature differences indicate about heat transfer?
To answer this question, you will explore
Heat Transfer
The First and Second Laws of Thermodynamics
Heat Transfer
EXPLORING THE TOPIC
Heat Transfer
CONSUMER CONNECTION
CONSUMER
CONNECTION
Materials that do not conduct heat well are called thermal insulators. We use them in the walls and ceilings of our homes to slow down the transfer of heat from our homes in the wintertime and into our homes in the summertime. A thermos bottle is composed of several layers of insulating material, often with a vacuum in the middle, to limit heat transfer and to keep cold liquids cold and hot liquids hot.

There are three main ways heat is transferred: conduction, convection, and radiation.
Conduction takes place when a substance transfers heat to another substance or object that it is in contact with. For example, the handle on a frying pan gets hot because the frying pan is hot.
Convection takes place when a warm substance changes location, such as when warm air rises.
Radiation takes place when electromagnetic waves carry energy from an energy source, such as the Sun.
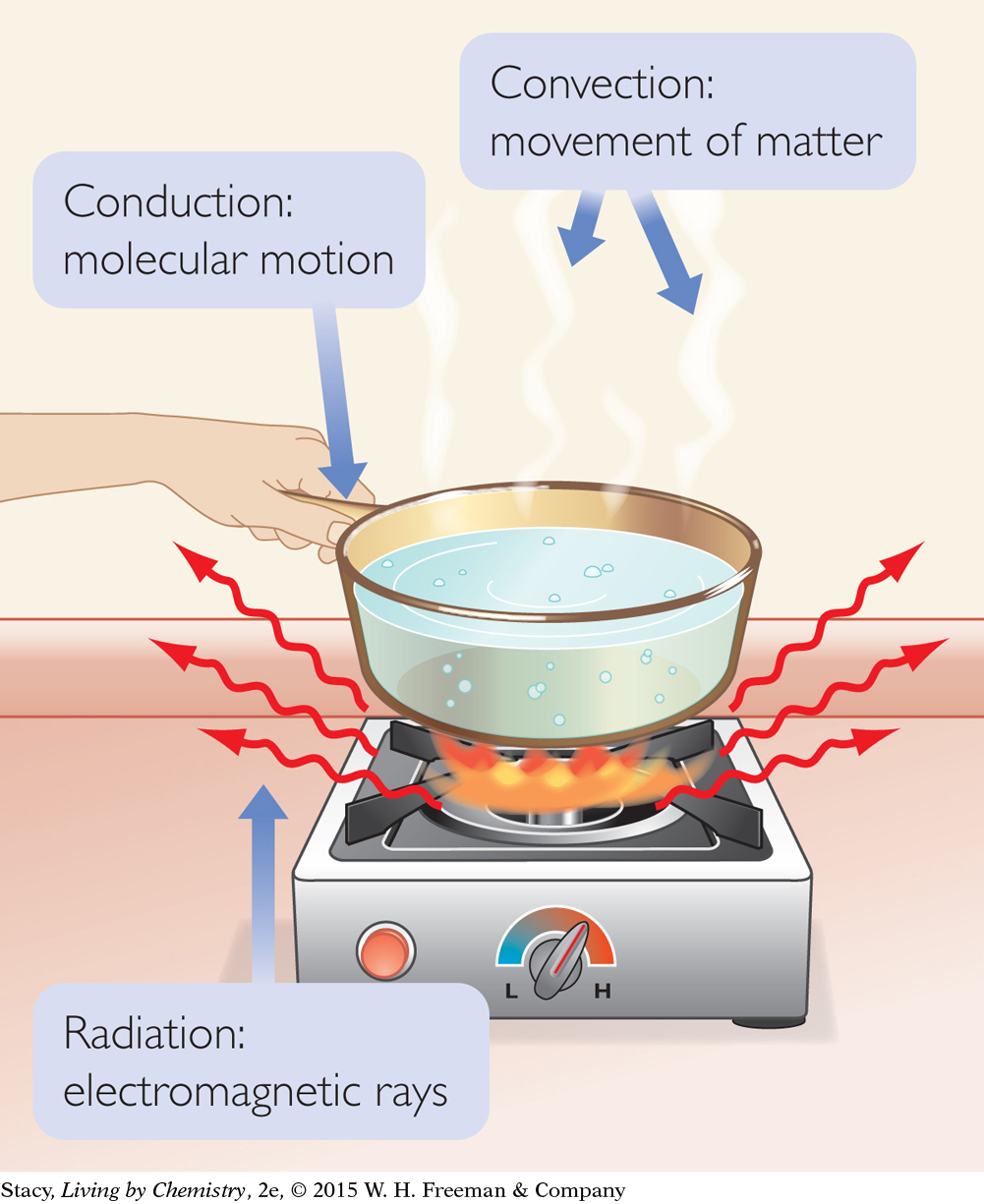
SYSTEM AND SURROUNDINGS
Consider each of the three illustrations. Assume water is the system. Try to identify the direction of heat transfer, the type of heat transfer, and the surroundings.
|

Water boils on the stove, producing water vapor. When the water vapor comes in contact with the cold pane of glass it condenses and forms drops.
|
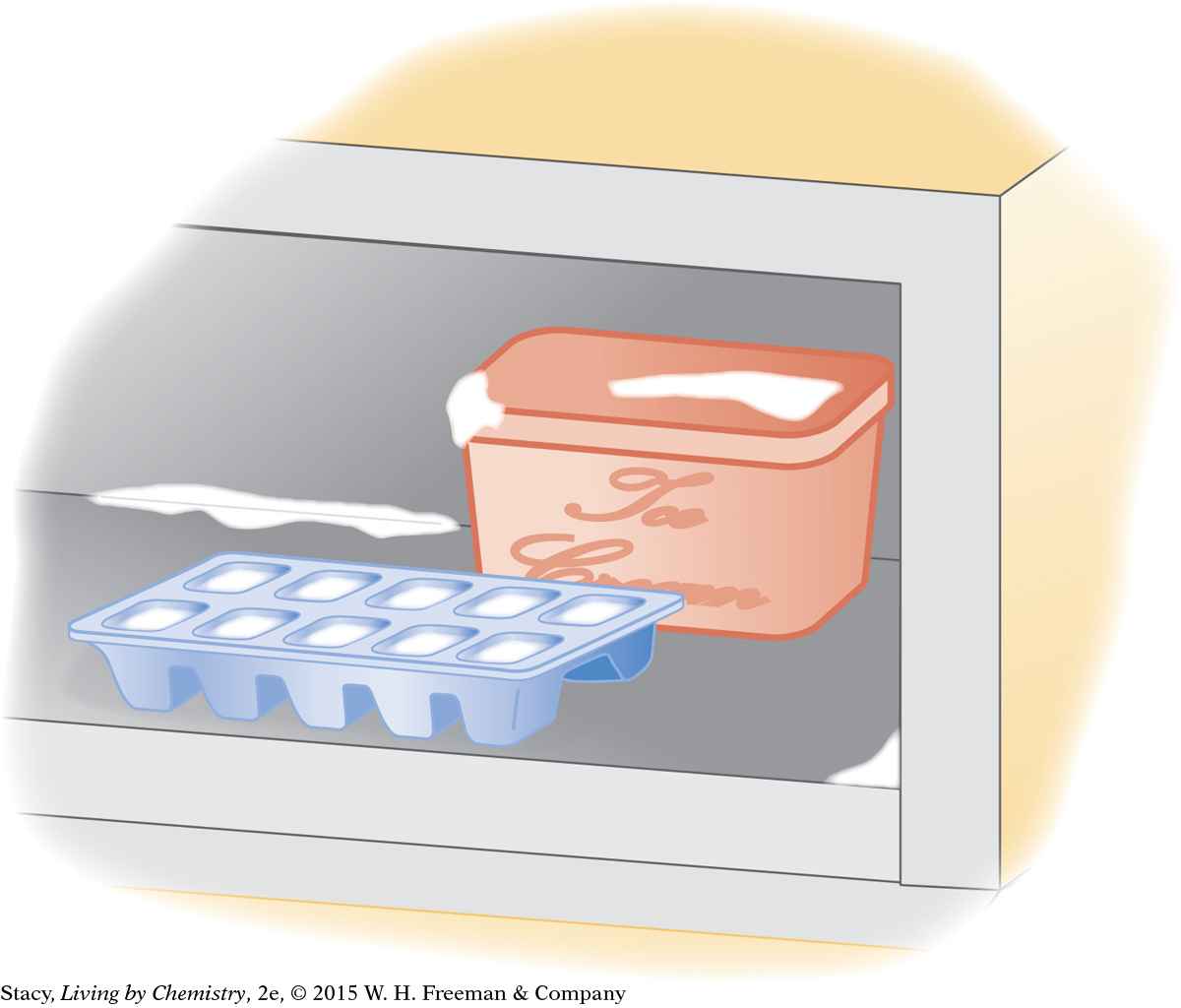
Water is placed in the freezer to make ice cubes.
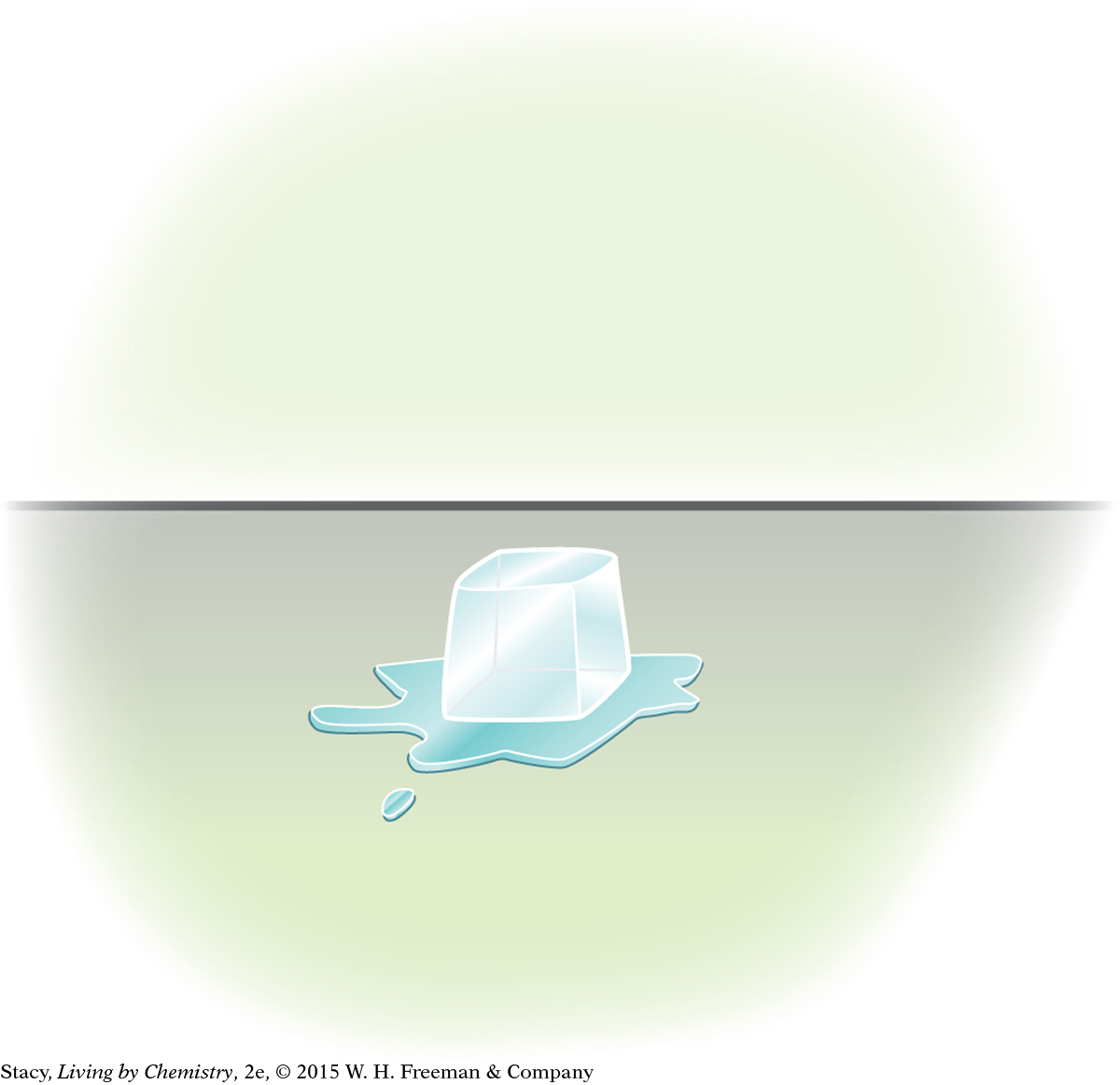
An ice cube is on a table and melts.
|
 In the first illustration, water molecules are in the form of both water vapor and liquid water. For the water vapor, the surroundings include the stove, the pot, the window, and the air in the room. Heat transfers from the stove to the pot and then to the water by conduction, then to the air in the room by convection. Heat also transfers from the water vapor to the window by conduction.
In the first illustration, water molecules are in the form of both water vapor and liquid water. For the water vapor, the surroundings include the stove, the pot, the window, and the air in the room. Heat transfers from the stove to the pot and then to the water by conduction, then to the air in the room by convection. Heat also transfers from the water vapor to the window by conduction.
 In the second illustration, water takes the form of both liquid and solid. For the liquid water in the ice cube tray, the surroundings include the ice cube tray, the freezer, and the air in the freezer. Heat transfers from the water to the surroundings by conduction. When enough heat energy transfers from the liquid water to the interior of the freezer, the water freezes and becomes solid.
In the second illustration, water takes the form of both liquid and solid. For the liquid water in the ice cube tray, the surroundings include the ice cube tray, the freezer, and the air in the freezer. Heat transfers from the water to the surroundings by conduction. When enough heat energy transfers from the liquid water to the interior of the freezer, the water freezes and becomes solid.
 For the ice cube on the table, the surroundings include the table and the air in the room. Heat transfers to the ice cube by conduction. When enough energy is transferred into the ice cube on the table, it melts.
For the ice cube on the table, the surroundings include the table and the air in the room. Heat transfers to the ice cube by conduction. When enough energy is transferred into the ice cube on the table, it melts.
HOT AND COLD
The sensation of hot or cold depends on the direction of heat transfer. For example, if you hold an ice cube, your hand will feel cold as heat transfers from your hand to the ice cube. If you hold a cup of hot tea, your hand will feel warm as heat transfers from the hot tea to the cup and then to your hand. However, “hot” and “cold” are relative.
Important to Know
There is no such thing as “cold transfer.” Cold is the experience of heat transfer away from our bodies.
Just because something is at a low temperature does not mean it will feel cold. For example, if your hands are cold because you have been out in the snow all day, even holding them under cold water can feel painfully hot because the water is at a higher temperature than your hands. The experience of hot or cold depends on whether energy is being transferred to or from the observer.
THERMAL EQUILIBRIUM
RECREATION CONNECTION
RECREATION
CONNECTION
Hypothermia occurs when the body loses heat faster than it can generate heat. A body temperature below 95 ºF (35 ºC) is considered dangerous. People who are outdoors in winter without enough protective clothing, or who accidentally fall into very cold water, may develop hypothermia. Hot liquids and external heat sources can help a person recover from hypothermia.

Heat transfer occurs between two substances that are in contact with each other until there is no longer a temperature difference between them. If you take a pot of hot soup off the stove and place it on the counter, it will transfer heat to the air and the countertop. The soup (and the metal pot) will continue to transfer heat until they are at the same temperature as the room.
If two objects at different temperatures are in contact with one another long enough, the two substances will reach the same temperature. When they reach the same temperature, they are at thermal equilibrium.
Big Idea
Big Idea
The direction of heat transfer is always from a hotter substance or object to a colder one.
The First and Second Laws of Thermodynamics
The First and Second Laws of Thermodynamics
The study of heat transfer is called thermodynamics. The principles behind thermodynamics have been summarized into scientific laws that are consistent with observations of a large number of systems.
The first law of thermodynamics states that energy is always conserved. The heat transferred from a hotter object is equal to the heat transferred to the colder objects surrounding it. There is no loss of energy. Likewise, you cannot create energy. It can only be transferred from one place to another place, and it can change form, such as when solar energy is converted to electrical energy.
Big Idea
Big Idea
Energy is conserved. It cannot be created or destroyed.
The second law of thermodynamics states that energy tends to disperse, or spread out. Energy transfers from hotter objects to colder objects until the temperature evens out. It does not transfer from a cold object to a hot object because this would concentrate the energy in one place rather than spread it out. This concept helps to explain why you can sit far away from a fire and become warmed by it. The energy from the fire disperses, or spreads out, from its source.
Another way to explain this concept is to say that the entropy of a system tends to increase over time. This means that energy and matter have a natural tendency to become more dispersed and disordered rather than more collected and ordered. For example, when you open a bottle of perfume, the molecules disperse throughout the room. The molecules will not naturally collect back into the bottle.
Big Idea
Big Idea
Energy tends to disperse.
LESSON SUMMARY
LESSON SUMMARY
What do temperature differences indicate about heat transfer?
KEY TERMS
thermal equilibrium
first law of thermodynamics
second law of thermodynamics
entropy
The sensory experience of hot or cold depends on the direction of heat transfer. If your hand is at a higher temperature than what you are touching, the object will feel cold. If your hand is at a lower temperature, the object will feel warm. Heat is always transferred from a hotter object or to a colder one. Heat transfer is associated with phase changes. An ice cube melts because heat is transferred into it from the surroundings. If objects remain in contact with each other, heat is transferred until objects are at the same temperature. This balanced state is referred to as thermal equilibrium. Two laws of thermodynamics are related to these observations: The first law states that energy is conserved, and the second law states that energy tends to disperse, or spread out.
Exercises
Reading Questions
Why does an ice cube feel cold to your hand?
What is thermal equilibrium?
Reason and Apply
Is energy transferred to a liquid or from a liquid when it evaporates? When it solidifies?
Is energy transferred to a gas or from a gas when it condenses?
What is evaporation and why is it a cooling process? What cools during evaporation?
What determines the direction of heat transfer?
Do humans ever reach thermal equilibrium with the surrounding air? Explain.
Provide evidence to support the first law of thermodynamics.
Provide evidence to support the second law of thermodynamics.
Explain why a campfire burns out. Explain why the ashes eventually cool to the temperature of the surrounding air.
Could there ever be a situation in which an ice cube actually heats up something else? Explain.