LESSON 99: Where’s the Heat?
THINK ABOUT IT
Water boils at 100 ºC, at 1 atmosphere pressure. Once it reaches that temperature, liquid water does not get any hotter, no matter how big the flames are under its container or how much you heat it. If the temperature of the boiling water is not changing, where is all that heat going?
What happens to the heat during a phase change?
To answer this question, you will explore
The Heating Curve of Water
Heat and Phase Changes
The Heating Curve of Water
EXPLORING THE TOPIC
The Heating Curve of Water
Imagine that you have an ice cube that you have just removed from the freezer. The temperature of the freezer is below zero. The graph shows the changes in temperature as you heat the ice cube from below 0 ºC to above 100 ºC. The x-axis represents time. Because the water is being heated steadily, as time goes by, more and more heat is transferred to the water. This graph is the heating curve of water.
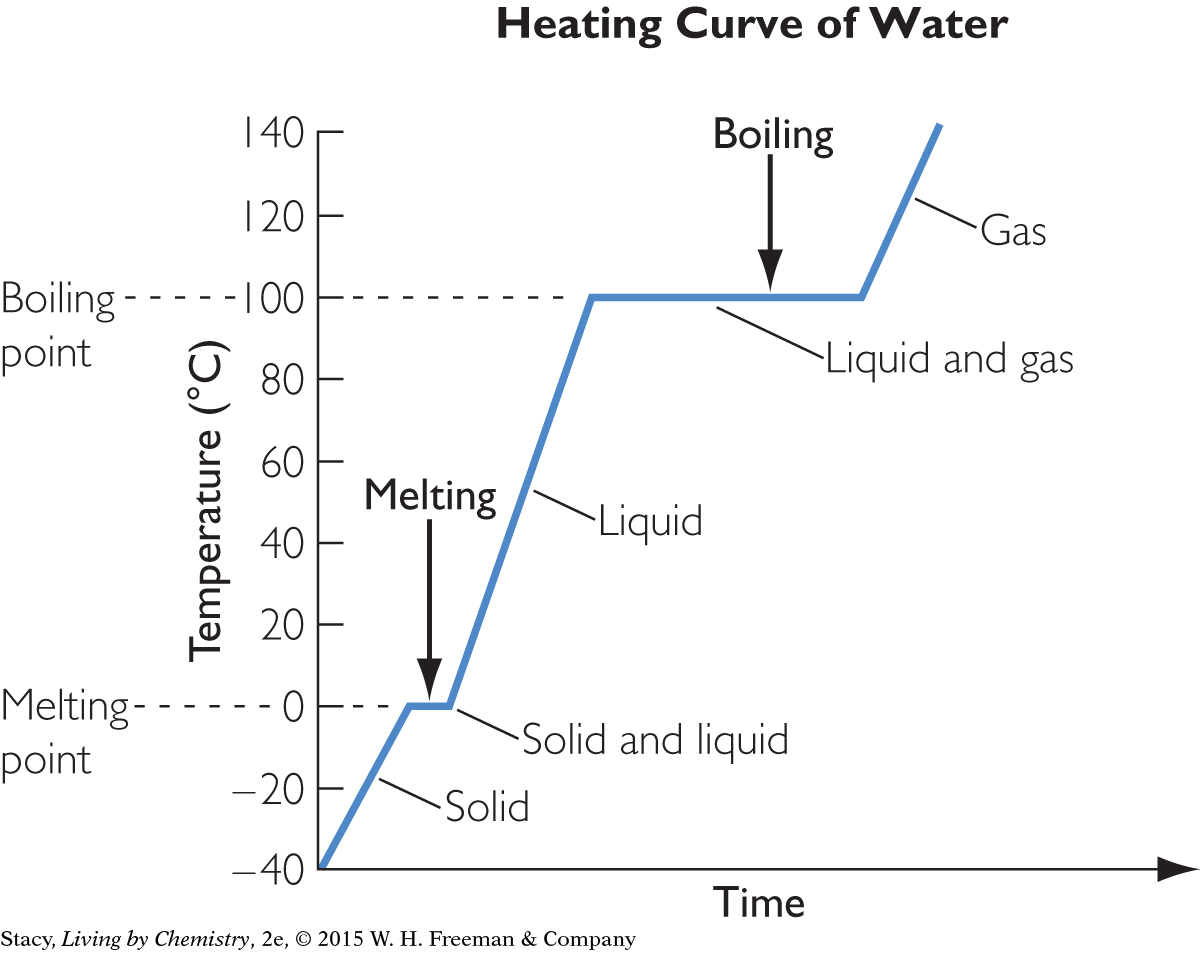
As you can see, the heating curve of water is a series of steep inclines with flat horizontal parts in between. Along the horizontal parts, energy continues to be transferred into the water, but the temperature does not change. The flat lines occur when melting or boiling is taking place, at 0 ºC or 100 ºC.
Note that whenever the temperature is not changing, two phases are present. As soon as only one phase—solid, liquid, or gas—is present, the temperature is able to change again. Something is happening to the energy going into the water during phase change.
Heat and Phase Changes
Heat and Phase Changes
During melting and boiling, there is no doubt that energy is going into the substance. However, this energy is doing something besides making the molecules move faster. Remember, temperature is a measure of the average kinetic energy of the molecules. If the temperature is not going up, then the molecules are not moving any faster on average. Where is the energy going?
The answer lies in the intermolecular forces. These attractive forces hold particles in a solid or a liquid close to each other. When heat is transferred into a substance, like ice, the molecules in the ice move faster and the temperature rises. Once the temperature rises to 0 ºC, the ice begins melting. When heat is transferred into melting ice, the average kinetic energy does not change, and the temperature does not rise. Instead, the energy transferred weakens the attractive forces between the molecules so that they can wiggle and rotate. Once the ice has melted completely, the energy transferred causes the molecules in the liquid to move faster, and the temperature rises once again.
During the boiling process, all of the energy transferred is used to completely break the intermolecular attractions so that molecules can move freely as a gas. As a result, no temperature change is observed during boiling.
Big Idea
Big Idea
Heat transfer does not always result in a temperature change.
When a substance changes phase from solid to liquid or from liquid to gas, a certain amount of energy must be supplied to overcome the molecular or ionic attractions between the particles. This energy is changing the solid into the liquid or the liquid into a gas, without changing the temperature. For example, the heat required to change 1 gram of a substance from a liquid into a gas is called the heat of vaporization. The heat required to change 1 gram of a substance from a solid to a liquid is called the heat of fusion.
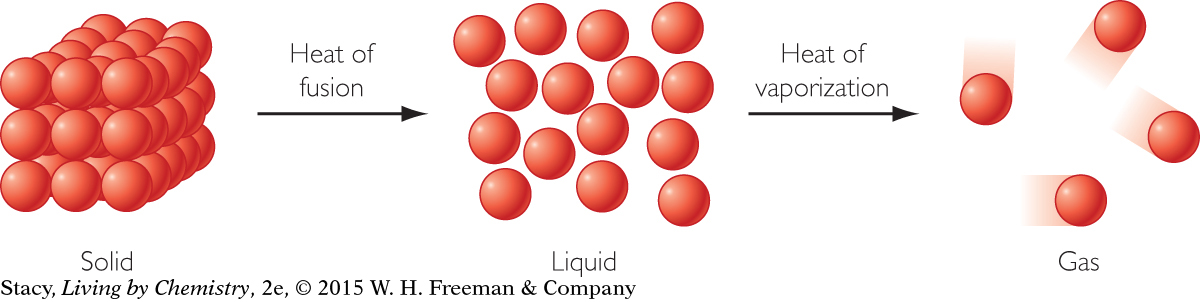
When a substance changes phase from a solid to a liquid, or from a liquid to a gas, energy is transferred to the substance from the surroundings. When a substance changes phase from a gas to a liquid, or from a liquid to a solid, energy is transferred from the substance to the surroundings.
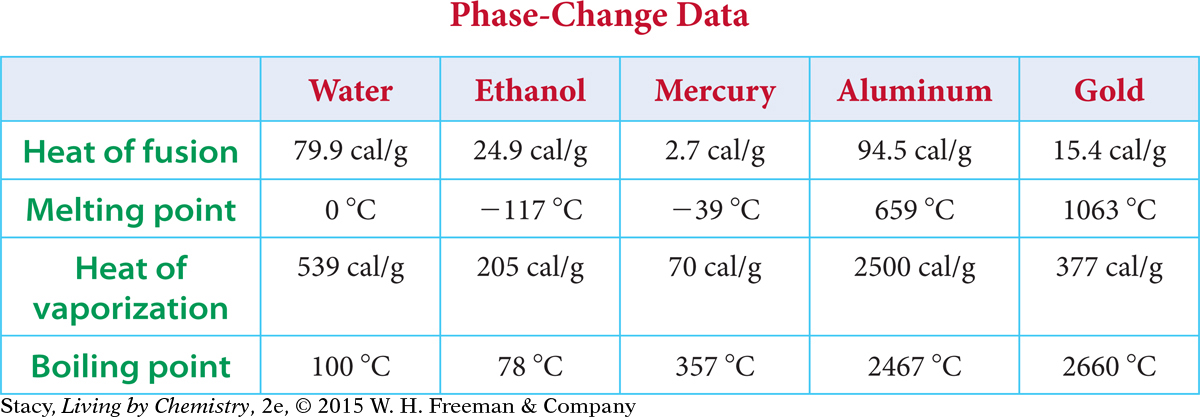
The table below shows some phase-change data for five common substances. Notice that it takes very little energy per gram to melt mercury (2.7 calories per gram), whereas it takes a great deal of energy to melt aluminum (94.5 calories per gram). Substances also require significantly more energy to change into gases than to melt.
Example
Heat of Fusion
You place 11 g of ice at 0 ºC into 100 g of liquid water at 20 ºC in an insulated water bottle. After a few minutes, the ice melts completely. Calculate the final temperature of the water in the bottle.
Solution
The heat of fusion of water given in the table is 79.9 cal/g. This means that, to melt 11 g of ice, 79.9 cal/g · 11 g, or 879 calories, are transferred. This energy is transferred from the 100 g of liquid water in the bottle. Use the equation for heat transfer to determine how much this amount of energy changes the temperature.
q = mCpΔT
− 879 cal = (100 g)(1 cal/g · °C)ΔT
ΔT = − 8.8 °C
The final temperature of the 100 g of water is 20 ºC − 8.8 ºC, or 11.2 ºC. But there are also 11 g melted ice at 0 ºC, so you need to find the weighted average of all the water in the bottle.
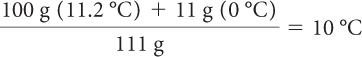
So, the final temperature of the water in your insulated water bottle will be around 10 ºC.
LESSON SUMMARY
LESSON SUMMARY
What happens to the heat during a phase change?
KEY TERMS
heat of vaporization
heat of fusion
Heat transfer does not always result in a temperature increase. During a phase change, the transfer of energy into a substance does not cause any rise in temperature. Instead, this energy is used to overcome the attractions between atoms and molecules.
The heat of fusion is the energy required to change a substance from solid to liquid. The heat of vaporization is the energy required to change a substance from liquid to gas.
Exercises
Reading Questions
Why doesn’t the temperature of water change during boiling?
What is heat of vaporization? Use a substance from the phase-change data table on page 507 as an example.
Reason and Apply
Use the table of phase-change data on page 507 to sketch a heating curve for mercury.
A jeweler pours liquid gold into a mold to make a 6 g wedding ring. How much energy is transferred from the ring when it solidifies? What temperature is the gold at the instant all of its atoms become solid?
Which of the following processes are exothermic? Explain your thinking.
boiling ethanol
freezing liquid mercury
subliming carbon dioxide
How much energy is required to melt these quantities of ice?
56 g
56 mol
If you transfer 5000 cal, how many grams of ethanol can you vaporize?
How much energy do you need to transfer to raise the temperature of 150 g of aluminum from 20 ºC to its melting point?
How much energy do you need to transfer to melt 150 g of aluminum?
You place 25.0 g of ice at 0 ºC into 100 g of liquid water at 45 ºC. The final temperature is 20 ºC. Show that energy is conserved.