LESSON 102: Counting Calories
THINK ABOUT IT
Heating a beaker of water with a burning potato chip seems like an unusual way to determine the number of Calories in this snack food. However, this is very close to the actual procedure used by nutritionists.
How does a calorimetry experiment translate into Calories?
To answer this question, you will explore
Calorimetry in the Lab
Calorimetry Calculations
Calorimetry in the Lab
EXPLORING THE TOPIC
Calorimetry in the Lab
Suppose that you wanted to measure the amount of energy transfer from combustion of a cashew nut. Because the digestive processes of the human body cannot be duplicated in your classroom, the next best approach is to determine the energy that can be transferred when food reacts directly with oxygen. To do this, you burn the food item and transfer the energy released due to combustion to another substance. Many substances can be used, but the most common one to use is water.
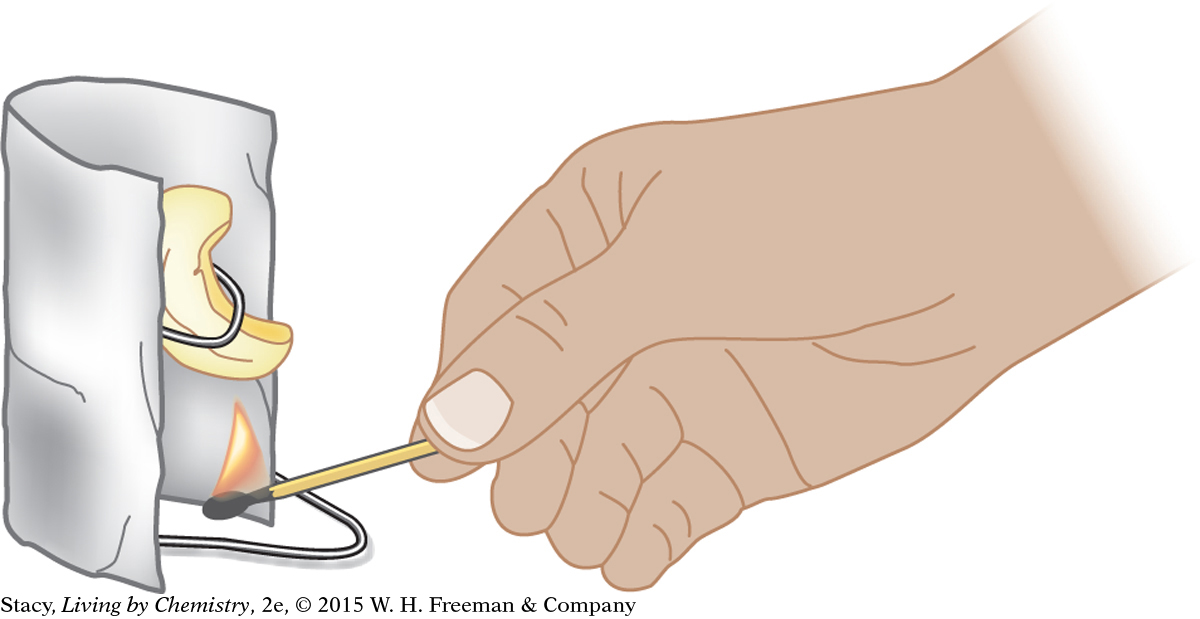
One feature of this type of experiment that is difficult to control in the lab is heat transfer to the surrounding air and equipment. Not all of the energy from burning is transferred directly to the water. Some thermal energy is transferred to the container, to the air, and even to you. One way to control this loss of energy is to burn the food sample very close to the bottom of the water container. Another way is to create some sort of shield to keep the energy transfer focused on the water. All of these details must be taken into account when designing a procedure.
Calorimetry Calculations
Calorimetry Calculations
There are three measurements and one property that help you to determine the energy transferred during a calorimetry procedure.
Data needed:
Mass of water heated in grams; 1 mL = 1 g
Temperature change of the water in degrees Celsius, ΔT (delta T)
Mass of fuel burned in grams
Specific heat capacity of water = 1.00 cal/g °C at 25 °C and 1 atm pressure
With the first two pieces of data, you can calculate how many calories of thermal energy are transferred to the water. Recall that the equation for heat transfer is
q = mCp ΔT
where Cp is the specific heat capacity of the substance being heated.
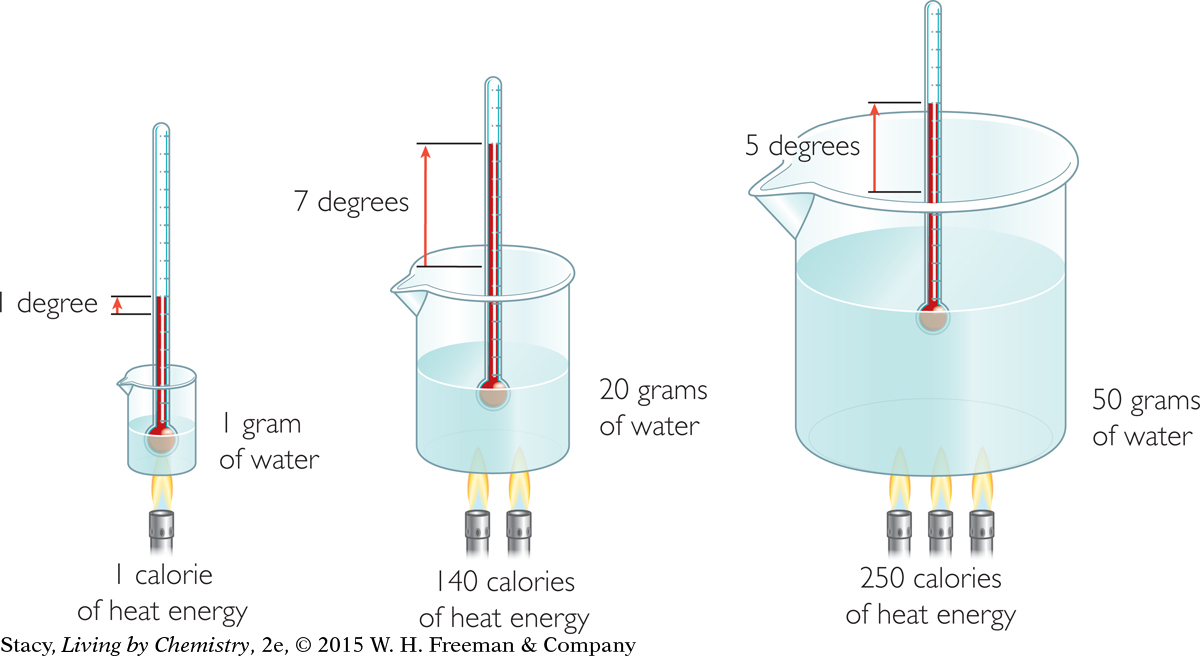
The illustration shows that if you multiply the change in temperature by the mass of the water, you can calculate the number of calories of heat transferred to the water. This is because the specific heat capacity of water is 1.00 cal/g · °C.
Example 1
Calories from a Cashew
These data were collected from the burning of one cashew. How much thermal energy was transferred?
Initial T of water = 19.0 °C
Final T of water = 34.5 °C
Volume of water = 30.0 mL
Solution
First, figure out the temperature change.
Subtract the initial temperature from the final temperature.
ΔT = 34.5 °C – 19.0 °C = 15.5 °C
Since 1 mL of water is equal to 1.0 g of water, the mass of the water heated is 30.0 g.
Use the equation for heat transfer.
Substitute values and solve.
q = mCp ΔT = 30.0 g(1.00 cal/g · °C)15.5 °C = 465 cal
One cashew transferred 435 cal of energy to the water. To compare different foods, it is necessary to figure out the calories per gram of food burned.
Example 2
Calories per Gram of Cashew
How much energy per gram was released by the combustion of the cashew?
Initial mass of cashew = 0.66 g
Final mass of cashew = 0.06 g
Solution
Figure out the mass of the cashew burned.
Subtract final mass from initial mass.
Mass of cashew burned = 0.66 g – 0.06 g = 0.60 g
Determine the number of calories per gram.
465 cal/0.60 g = 775 cal/g
The number of calories per gram is also called the food energy density. Cashews have an energy density of 775 cal/g of cashew burned. Remember, these are chemist’s calories. To convert them to food Calories, you must divide by 1000 to get 0.775 Cal/g.
The actual amount of energy a human obtains after the digestive processes are completed is about 85% of the Calorie content listed on nutrition labels. Fats have high energy densities, around 9 Cal/g. Sugars and proteins are around 4 Cal/g.
COMPARING FOODS
In the real world, nutritionists use calorimeters to figure out the Calorie content of foods. The word content is misleading. The food does not actually contain the Calories. The Calorie content represents the energy transferred specifically by combustion of these compounds.
Bomb calorimeters minimize the heat transfer to the surroundings, and the results are much more precise than our lab experiments. The method of calculation is very similar to the one used here. If a bomb calorimeter is used, a cashew would be found to have closer to 5500 chemists’ calories per gram, or 5.5 Cal/g. Our experimental results came up with 775 cal/g. There is a lot of error in the classroom procedure.
LESSON SUMMARY
LESSON SUMMARY
How does a calorimetry experiment translate into Calories?
Different fuels transfer different amounts of energy during a combustion reaction. Data about the temperature change, fuel mass, and water volume from a calorimetry procedure can be converted into Calories. Using the specific heat capacity of the substance that was heated by the reaction allows you to calculate the exact number of calories transferred by the combustion of the fuel. This provides you with a measure of the amount of thermal energy “contained” in a fuel. The number of calories transferred from a substance that burns depends on the identity of the substance and its mass.
Exercises
Reading Questions
Name three possible sources of error in a calorimetry experiment.
Why is it important to know the specific heat of the substance being heated by the combustion of a fuel?
Reason and Apply
Could you use ethanol instead of water in a calorimetry experiment? Explain.
The experimental value for the Calorie content of a cashew is 0.775 Cal/g. The actual value provided on the package label is 5.5 Cal/g. Determine the percent error.
A cereal flake is burned under a beaker containing 25 mL of water. If the water temperature goes up 6 °C, how many calories of energy were transferred to the water?
Fuel pellets are used in modern energy-saving wood stoves. If the pellets used for these stoves release 742 cal/g, how many calories of energy will be released by combustion of an entire 40 lb sack of pellets?
Examine the illustration on page 520. Use a kinetic molecular view to explain why it takes more calories of heat to raise the water temperature in the third beaker by 5 °C than it does to raise the temperature of the water in the second beaker by 7 °C.
The calorie content of a peanut is measured by burning it beneath a can of water and measuring the temperature change of the water. Which of these is a possible source of error?
The initial mass of the peanut is measured incorrectly.
Some of the heat of combustion is transferred to the air.
Some unburned remnants of the peanut are lost before finding the final mass.
All of the above.
None of the above.