LESSON 3: What’s the Matter?: Defining Matter
THINK ABOUT IT
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Gold has several special properties. It is shiny and it does not rust or tarnish. It is smooth, bendable, easy to dent, and even a small piece of gold is surprisingly heavy.

People tend to value gold over other substances. You don’t often see someone wearing aluminum jewelry or putting coal in a high-security bank vault. What is it about gold that makes it unique? Is it possible to create gold from another substance?
This lesson begins to explore the nature of matter as the first step toward proving whether you can or cannot create gold. After all, chemistry is the study of matter and its properties.
What is matter?
To answer this question, you will explore
Defining Matter
Is It Matter?
Measuring Matter
Defining Matter
EXPLORING THE TOPIC
Defining Matter
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Viruses are so small that the length of a virus or other microorganism is measured in microns. A micron is equal to 0.001 millimeter. The viruses in this image have been magnified 10,000 times and colorized to make them easier to see.
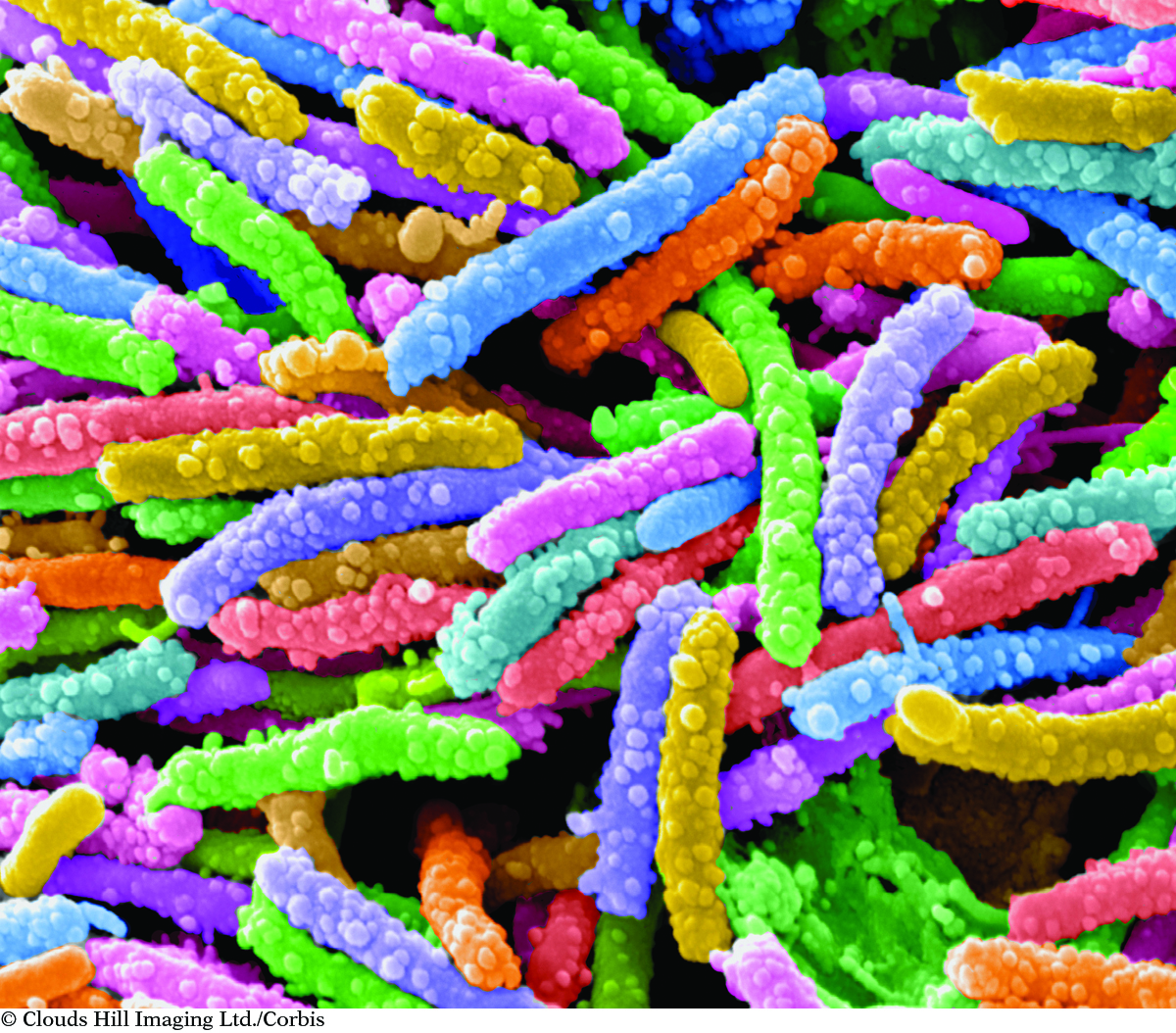
Matter is the word chemists use to refer to all the materials and objects in the world. Your desk, this book, and the paper and ink in the book all are matter. These are all things you can see or feel. However, your senses alone are not always enough to tell you if something is matter. For instance, you cannot see the virus that gave you a cold, but it is matter. Conversely, you can see shadows on the ground cast by the light from the sun, but they are not matter.
A gold ring, the ink in this book, and a virus each have substance, which means they are made out of material, or “stuff.” The amount of substance, or material, in an object is called mass. Mass is a property of matter that can be measured. So, although the virus has very little substance, it still has mass.
Another property that a gold ring, the ink in this book, and a virus have in common is that they take up space, which means they have dimensions. The amount of space something takes up is called volume and is also a property of matter that can be measured.
So, matter is anything that has mass and volume. You can also say matter is anything that has substance and takes up space. This explains why a virus is matter but a shadow is not.
Is It Matter?
Is It Matter?
It is easy to see that solids and liquids have mass and volume. When water is poured into a container, you can see how much space it takes up. When filled with water, the container has more mass. You can see this difference if you use a balance. On a two-pan balance, a cup with water will be lower than an identical cup with no water because it has more mass. Thus, water is matter.
When identifying matter, gases can be misleading. For example, most of the time you do not see or feel the air around you and it may seem as if nothing is there. However, when you fill a balloon with carbon dioxide gas, you can see that the carbon dioxide gas inside occupies space. On a two-pan balance, the pan with a balloon filled with carbon dioxide gas will be lower than the pan with an empty balloon because the balloon filled with carbon dioxide gas has more mass. Therefore, gases do have mass and volume even though gases might be harder to detect than solids and liquids.
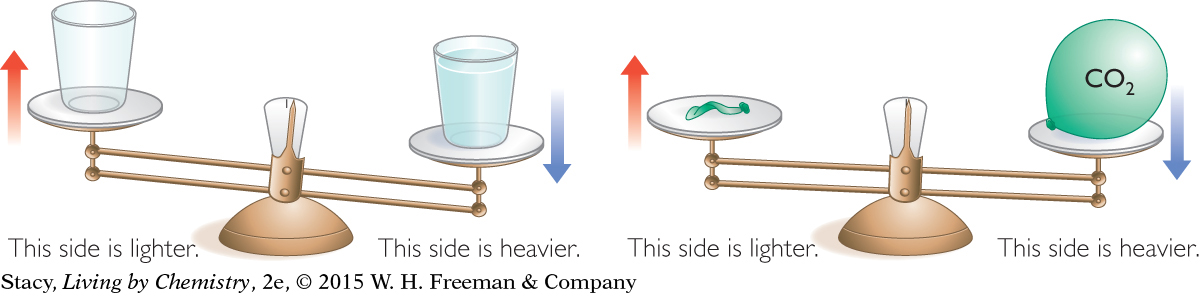
What about other things, like heat and sound? Are they matter? When you heat soup, it does not gain mass, and sound may “fill the room,” but it doesn’t have mass or volume. Sound is the movement of air against your eardrums. Without matter, sound can’t exist, but sound itself is not matter. Similarly, heat can’t exist without some form of matter, but heat itself is not matter. Both sound and heat are referred to as types of energy, and energy by itself is not matter. Likewise, feelings and thoughts are not matter, although you could argue that they require an interaction of matter and energy.
Example
Is It Matter?
Classify wind, music, and clouds. Are they matter? Explain your answer.
Solution
Wind is the movement of air. Air is matter, but the movement of air is not matter because movement has no mass or volume. Therefore, wind is not matter.
Music is sound, which is the movement of air against your eardrums. Music does not have mass or volume. Therefore, music is not matter.
Clouds are made of water droplets, which have mass and volume. Therefore, clouds are matter.
Measuring Matter
Measuring Matter
MEASURING MASS
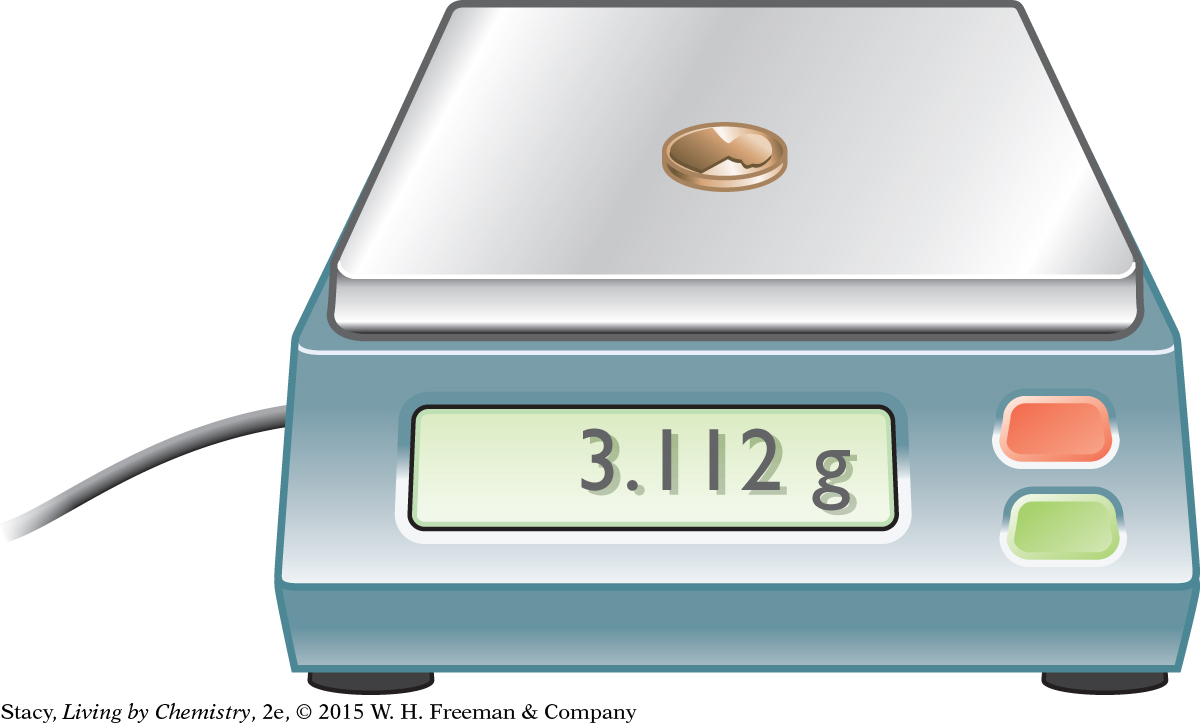
To find the mass of something, you weigh it. In everyday life, things are usually weighed in pounds, lb, or kilograms, kg. For example, you’ve probably weighed yourself and know approximately how many pounds you weigh. And when you go to the grocery store, you might buy a pound of butter or flour. However, chemists measure mass in kilograms, kg, and grams, g (1 kg = 1000 g). [For review of this math topic, see MATH Spotlight: SI Units of Measure on page A-0.]
In the chemistry classroom, an electronic balance is used to measure mass. The balance is precise and shows more digits (places) than you may need. You have to decide how many places to pay attention to when using this balance. For example, when you weigh yourself, you measure to the nearest kilogram. You would state your mass as 70 kg, not 69.903 kg. But if you are measuring the mass of a penny, you may want to be more exact. The mass of the copper penny to the nearest hundredth of a gram is 3.11 g. The mass to the nearest gram is 3 g.

This beaker measures to the nearest tenth of a liter.
Ken Karp Photography
|

This graduated cylinder measures to the nearest milliliter.
Ken Karp Photography
|
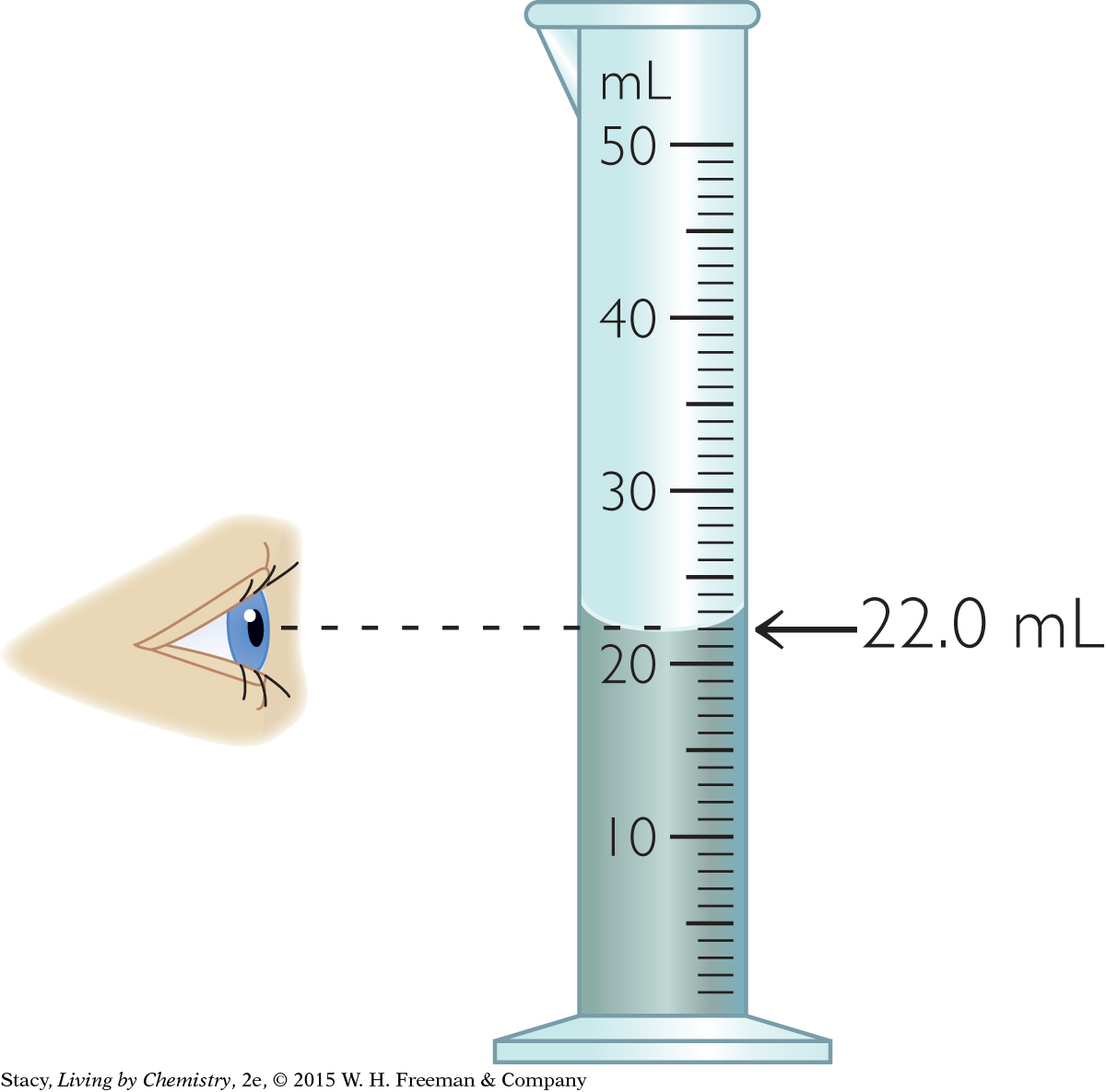
Read the measurement at the bottom of the meniscus. Make sure your eye is at the water level.
|
MEASURING VOLUME
You can measure the volume of a regularly shaped object by measuring its dimensions, such as length, width, and height, and using a mathematical formula. Volume measured this way is reported in cubic units, for example, cubic meters, m3, or cubic centimeters, cm3.
Chemists often measure the volume of gases and liquids in liters, L, and milliliters, mL (1 L = 1000 mL). The volume of a large soda bottle is 2 L, or 2000 mL. This is the volume of the bottle, and if the bottle is full, it is also the volume of liquid inside. Chemists use a variety of special containers for measuring volume. For approximate measurements, they use beakers. For more precise measurements, they use graduated cylinders. [For review of this math topic, see MATH Spotlight: Accuracy, Precision, and Significant Digits on page A-1.]
You can measure the volume of a sample of water by pouring it into a graduated cylinder and reading the number of milliliters, mL, on the side. However, the surface of the water is curved because of the way it adheres, or clings, to the sides of the cylinder. The curvature of the top of a liquid in a container is called a meniscus. It is most accurate to read the water level at its lowest point, the bottom of the meniscus, because most of the liquid is at this level. To get an accurate measurement, you must read the graduated cylinder at eye level.
LESSON SUMMARY
LESSON SUMMARY
What is matter?
KEY TERMS
matter
mass
volume
meniscus
Chemists seek to understand matter and its properties. Matter can be defined as anything that has mass and volume. Mass is the amount of substance or “stuff” in a material or object. Solids, liquids, and gases all have mass and volume and are classified as matter. To measure the mass of something, you use a balance. Volume is the amount of space taken up by matter. To find the volume of something, you can use a container such as a beaker or graduated cylinder, or you can calculate the volume using dimensions, such as length, height, and width.
Exercises
Reading Questions
Explain the difference between mass and volume.
Describe how you could prove that a bicycle is matter.
Reason and Apply
Someone might claim that air is not matter because you can’t see it. Write a paragraph showing how you would prove to this person that air is matter.
The Sun is considered matter, but sunlight is not considered matter. Explain why this is so.
Sit somewhere and observe your environment. From your observations, make a list of ten things that are matter and a list of ten things that are not matter. Explain your reasoning.
Give examples of five words that describe the movement of matter.