Chapter 1 Summary

CHAPTER 1
Defining Matter
SUMMARY
KEY TERMS
hypothesis
property
chemistry
matter
mass
volume
meniscus
water displacement
density
intensive property
extensive property
Alchemy Update
Can a copper penny be turned into gold through chemical processes?
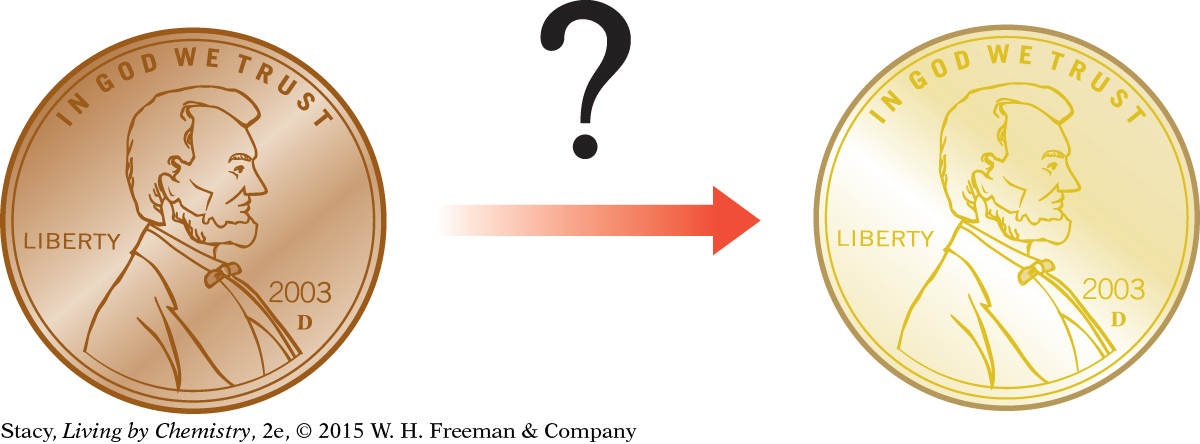
The “golden” penny looks like gold and has almost exactly the same volume as a true gold penny. However, the golden penny has a much lower mass than a true gold penny. This means the density of the “golden” penny is less than the density of gold. It cannot be a solid gold penny.
The concept of density has provided the evidence needed to confirm that the gold-colored penny is not actually made of gold. But if it is not gold, then what is it? Many questions remain to be answered. Is it still possible to make gold some other way? And what is it about gold that makes it gold?
REVIEW EXERCISES
Question 1.1
1. Explain how you would determine the volume of a powdered solid, a liquid, and a rock.
Question 1.2
2. Use your own words to define matter.
Question 1.3
3. Will an object with a higher density displace more water than an object with a lower density? Explain why or why not.
Question 1.4
4. How does the density of one penny compare with the density of two pennies?
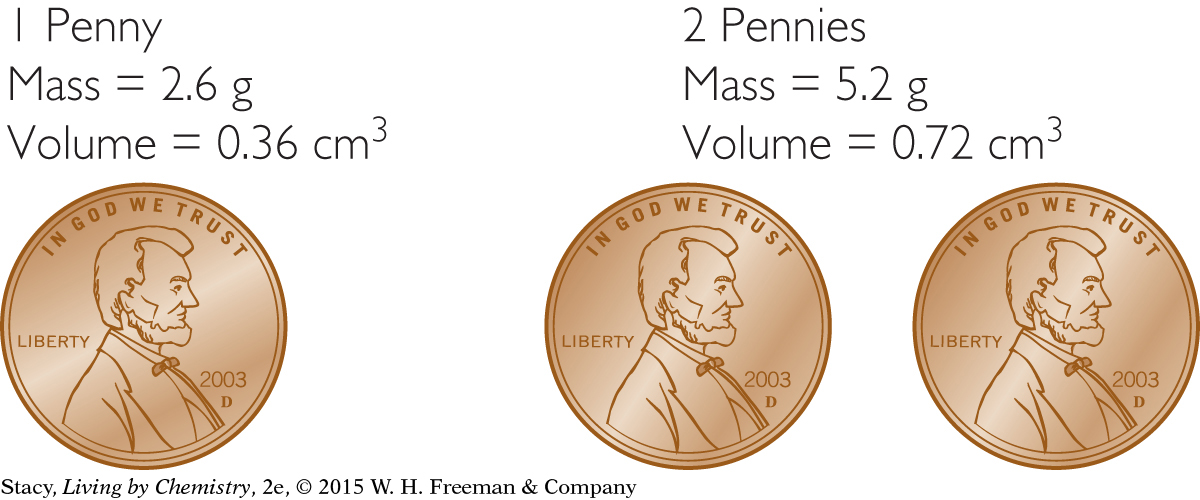
Question 1.5
5. A small pebble breaks off of a huge boulder. The pebble has the same density as the boulder. In your own words, explain how this can be true.
Question 1.6
6. Archeologists discover a silver crown in an ancient tomb. When they place the crown in a tub of water, it displaces 238.1 cm3 of water. The density of silver is 10.5 g/cm3. If the crown is really silver, what will its mass be?