Featured ACTIVITY: Make It or Break It
Featured ACTIVITY
Make It or Break It
Purpose

To explore bond energies and calculate the net energy exchange for several reactions.
Materials
ball-and-stick model kit
Questions
List at least two patterns you see in the table of average bond energies.
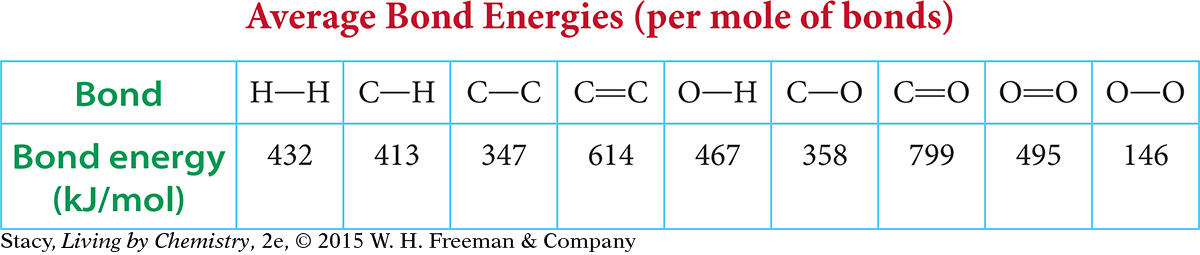
Use ball-and-stick models to create the reactants for this reaction.
Burning methane: CH4(l) + 2O2(g) → CO2(g) + 2H2O(g)
Rearrange the reactant models into carbon dioxide and water. Count how many of each type of bond you must break when this reaction takes place. Record the information in a table. Use the average bond energies to determine the amount of energy transferred to break all the bonds.
Count how many of each type of bond you must make to form the products. Record this information in your table. Use the average bond energies to determine the amount of energy transferred out when the new bonds are formed.
What is the net energy you expect to be transferred to the surroundings by this reaction?
The energy of reaction for exothermic reactions is normally expressed as a negative number. Why do you think it is negative?
Making Sense When a substance combusts, energy is transferred to the surroundings as heat and light. Where does that energy come from?
If You Finish Early What is the energy of reaction for 1 g of methane burned, in kilocalories per gram?