LESSON 106: Speed Things Up
THINK ABOUT IT

The Golden Gate Bridge in San Francisco is a national landmark. However, it is slowly eroding because of a chemical reaction in which iron in the steel beams combines with oxygen in the air to create iron oxide. This iron oxide falls away from the metal underneath. Is there a way to slow down the rate of this reaction?
How can you control the speed of a reaction?
To answer this question, you will explore
Reaction Rates
Catalysts
Reaction Rates
EXPLORING THE TOPIC
Reaction Rates
Some reactions proceed quickly and others proceed slowly. When a balloon full of hydrogen is ignited, it reacts rapidly, exploding into flame. The formation of iron oxide on steel bridges is a slower process called rusting.
| Combustion of hydrogen occurs rapidly. | 2H2(g) + O2(g) → 2H2O(g) |
| Rusting of iron occurs slowly. | 4Fe(s) + 3O2(g) → 2Fe2O3(s) |
Both of these reactions are exothermic, yet the rates of each reaction are very different.
You might notice that the reactants are both gases for the quicker reaction. Gases mix on a molecular scale, so there is a lot of contact between the reactants. In contrast, the slow reaction involves a solid. The reactants are in contact with one another only at the surface of the solid. There are many more iron atoms on the interior of the solid that are not exposed to oxygen and therefore do not react. When a layer of the iron oxide, rust, flakes off, the underlying iron is then exposed to oxygen and will react.
COLLISION THEORY
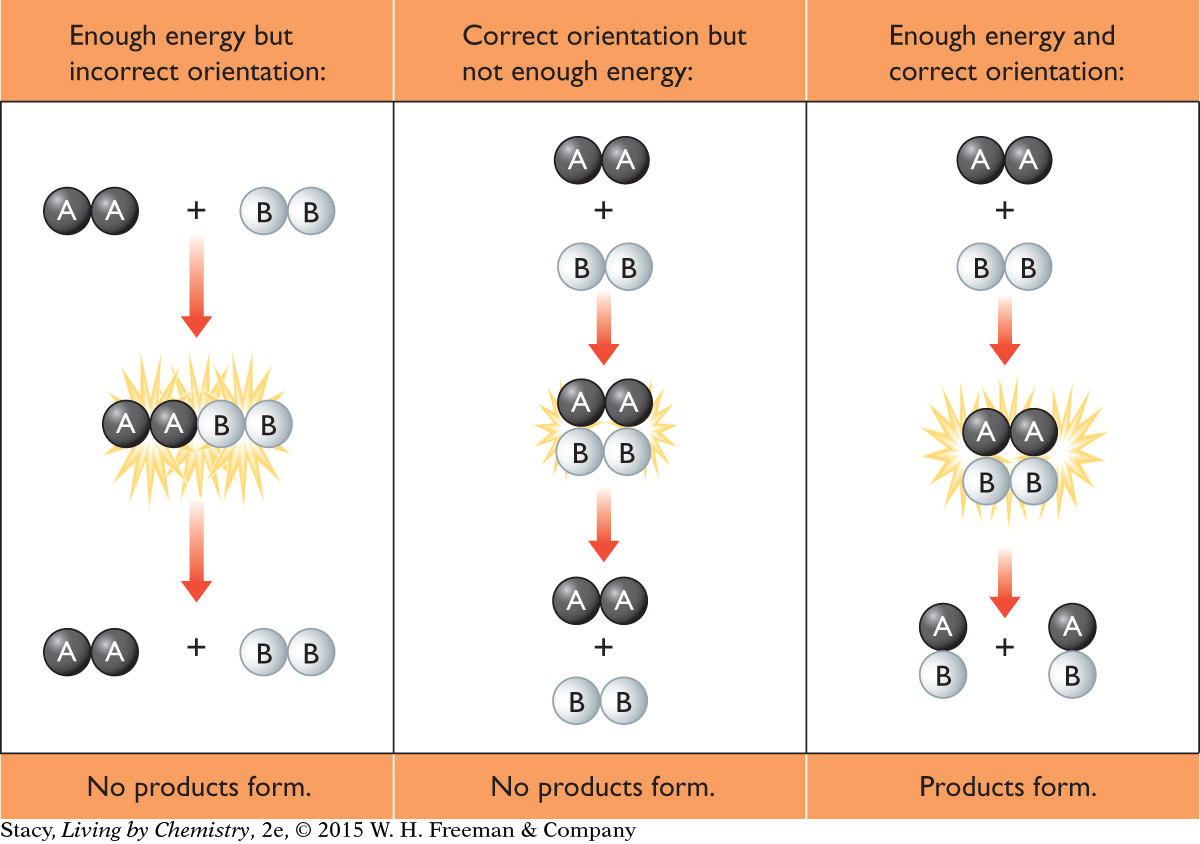
For a reaction to occur, the reactant particles must collide with enough energy to cause reactant bonds to break and product bonds to form. This energy is the activation energy of the reaction. Also, the reactant particles must collide in the correct orientation with one another. When this happens, the reactant particles are able to form a temporary arrangement called an activated complex, which facilitates bond breaking and bond making.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
Enzymes could be considered nature’s catalysts. They are biomolecules that speed up the rates of chemical reactions in living things. Enzymes are usually protein molecules. For example, the enzyme amylase in your saliva helps to break down sugar molecules. The enzyme lactase in the small intestine breaks down lactose. People with insufficient lactase have trouble digesting dairy products, which contain lactose.

So, for a reaction to occur, the reactant particles must collide in the correct orientation and with enough energy. If they do not, they will bounce apart and products will not form. Various factors can increase the frequency of collisions between particles and the energy of the collisions. These factors will speed up a reaction.
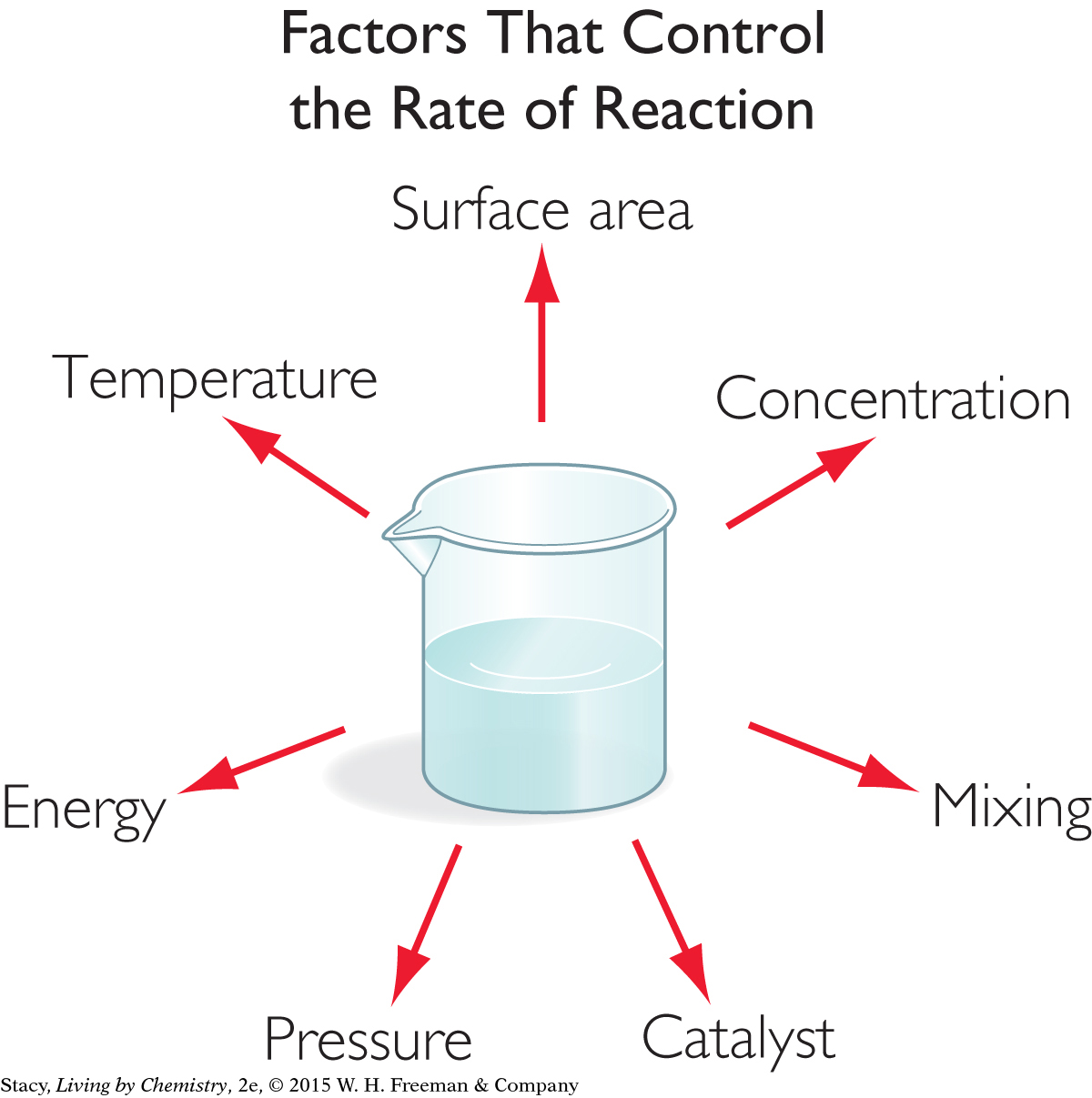
Surface area: The exposed surface area of the reactants can affect the rate of a reaction. For example, in combustion reactions, fuels with larger exposed surface area, like sawdust or twigs, burn more rapidly than fuels with smaller exposed surface area, like an entire tree or a wooden table.
Mixing: When reactants are mixed well, the reaction rate increases. This is related to the increased probability that the appropriate reactants will collide.
Temperature: When the temperature is raised, the average kinetic energy of the reactant particles is also raised. The particles move more rapidly and more collisions between particles are possible.
Concentration or pressure: Reactions usually proceed faster as the concentration of the reactants in solution increases or as the pressure of gases increases. Again, more reactants in a small volume result in more collisions.
Energy: Adding energy, such as microwave radiation or light, can increase the rate of a reaction.
Catalysts
Catalysts
Most of the methods of increasing the rate of reaction involve an increase in the number of collisions of the molecules or an increase in the speeds of the collisions. Another way to increase the rate of reaction is to add a catalyst to the reactants.
CONSUMER CONNECTION
CONSUMER
CONNECTION
The opposite of a catalyst is an inhibitor, a substance added to slow down a reaction. Sodium nitrite, ascorbic acid, and cinnamaldehyde are used to slow down rusting reactions. Inhibitors such as lemon juice, which contains ascorbic acid or 1-methylcyclopropene, can be added to fruit to slow down the reactions that cause browning.

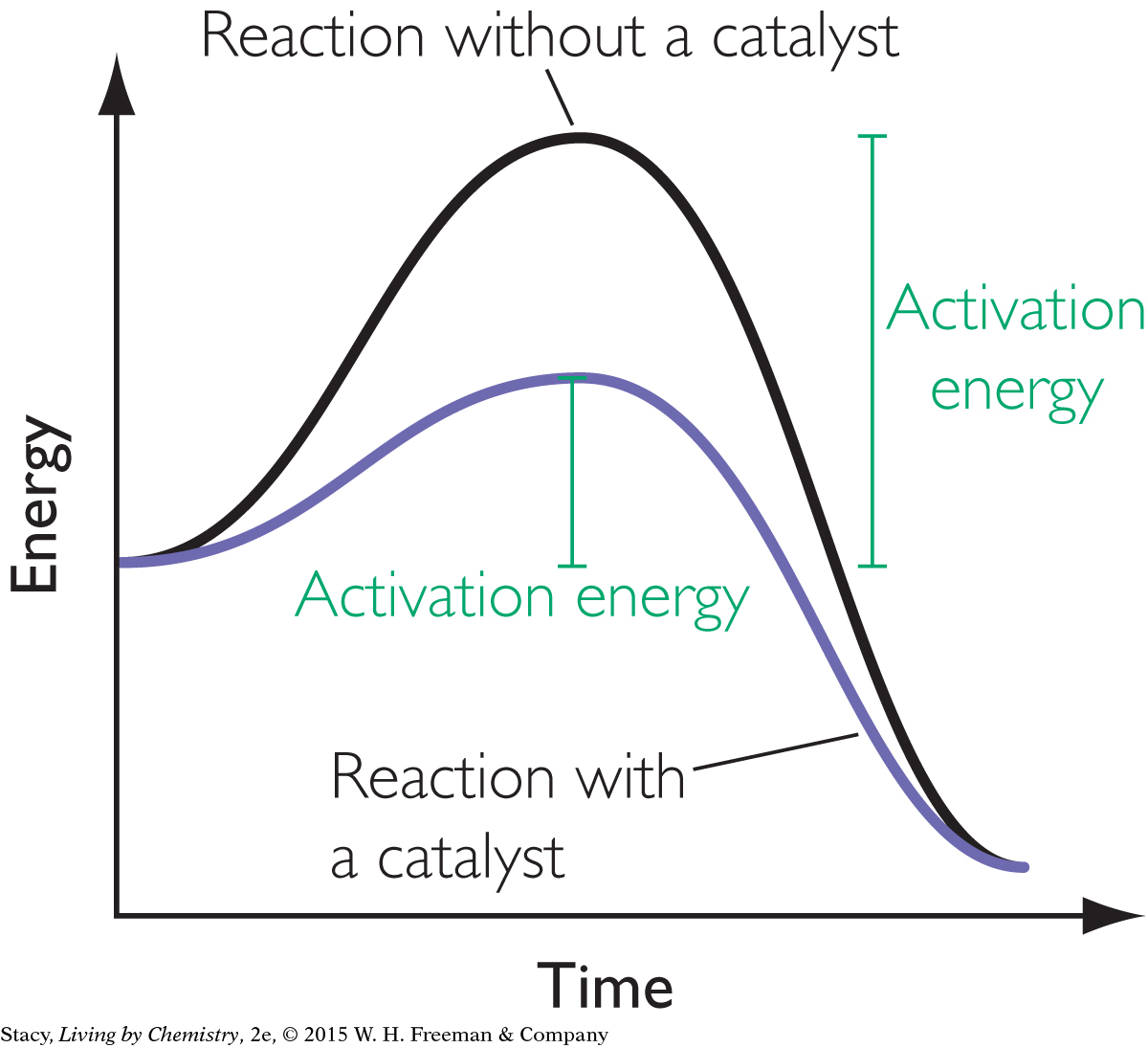
A catalyst is a substance that assists a reaction but is neither consumed nor permanently changed by the reaction. A catalyst lowers the activation energy for the reaction. In Unit 2: Smells, sulfuric acid was used to catalyze the reaction that produced sweet-smelling ester molecules.
Chemical reactions may go through a series of intermediate steps to get from reactants to products. Some catalysts work by changing the sequence of steps to shorten the reaction path. Catalysts may undergo chemical transformations during a reaction, but they always return to their original form at the end of the reaction.
Another way a catalyst works is to provide a surface on which the reaction takes place. A catalytic converter in a car provides a platinum surface where pollutants can react to form harmless gases.
The energy diagram shows the same reaction with and without a catalyst. The catalyst lowers the energy barrier for the reaction.
LESSON SUMMARY
LESSON SUMMARY
How can you control the speed of a reaction?
For molecules to react with each other, they must collide with a sufficient amount of energy for bond breaking or bond making. There are a number of ways to increase the rate of a reaction, such as increasing the temperature of the system or changing the concentration of the reactants. These methods increase collisions between reactants. One way catalysts increase the rate of a reaction is by lowering the activation energy to get the reaction started. Catalysts are substances that speed up a reaction but are not permanently changed or consumed by the reaction.
Exercises
Reading Questions
Name three things you could do to increase the rate of a reaction between two liquids.
Name three things you could do to increase the rate of a reaction between two solids.
Name two ways you could decrease the rate of a reaction.
Reason and Apply
What could you do to speed up the reactions listed here?
Lighting charcoal for a barbecue
Baking cupcakes
Dissolving sugar in water
Removing a stain from clothing
Which will burn faster, wood chips or a tree? Explain the difference in the rates of reaction.
Which will dissolve faster, a lollipop or powdered sugar? Explain the difference in the rates of reaction.
Which will react faster, a steel beam or powdered iron? Explain the difference in the rates of reaction.
Why do you think you can extinguish a flame with a carbon dioxide fire extinguisher?
What makes an explosion happen so quickly?
Research a particular enzyme in your body. Explain how the enzyme catalyzes a reaction in your body. The rate of a reaction can be affected by
The phase of the reactants.
The temperature of the reactants.
The presence of a catalyst.
All of the above.
None of the above.