LESSON 109: Pumping Iron: Heat of Formation
THINK ABOUT IT
554
With all the heat and light it gives off, fire is an obvious source of energy. Less obvious is the possible use of other exothermic chemical reactions as energy sources. Because the oxidation of metals is exothermic, it may be possible to use oxidation reactions to generate power.
How much energy is transferred during oxidation of metals?
To answer this question, you will explore
Heat of Formation
Reversing Oxidation
Heat of Formation
EXPLORING THE TOPIC
Heat of Formation
HISTORY CONNECTION
HISTORY
CONNECTION
Malachite, Cu2CO3(OH)2, a green stone, was probably the first source of copper metal that was extracted through heating. Malachite may have been used to color a piece of pottery that was then fired in a very hot oven, leaving behind globs of copper.

The oxidation of most metals is exothermic, just like the combustion of fuels. So, oxidation reactions are a potential source of energy for our daily lives. To explore the usefulness of metal oxidation as an energy source, we must examine and compare the amount of thermal energy transferred during these reactions.
COPPER VERSUS ZINC
Copper metal and zinc metal react with oxygen to form copper (II) oxide, CuO, and zinc oxide, ZnO.
| 2Cu(s) + O2(g) → 2CuO(s) | ΔH = –314 kJ |
| 2Zn(s) + O2(g) → 2ZnO(s) | ΔH = –696 kJ |
Notice that ΔH is negative for both reactions. As you recall, this indicates that the reactions are exothermic and heat is transferred to the surroundings. Also notice that in both cases the heat of reaction is associated with two moles of metal oxide being produced. So, the formation of one mole of each oxide results in the release of half as much energy.
The energy released when one mole of a compound is formed from elements is called the heat of formation, or ΔHf. Compare the heats of formation of copper (II) oxide, CuO, and zinc oxide, ZnO:
Heats of formation of copper (II) oxide: ΔHf (CuO) = –157kJ/mol
Heats of formation of zinc oxide: ΔHf (ZnO) = –348kJ/mol
These values indicate how much heat transfer to expect when one mole of each compound is formed from its elements. The formation of 1 mol of zinc oxide releases over twice as much energy as the formation of 1 mol of copper (II) oxide. You can find heats of formation values of some metal oxides in the table below. These values are determined under standard conditions, at 1 atm and 25 °C. Note that standard conditions are different from STP, standard temperature and pressure, which is 1 atm and 0 °C.
555
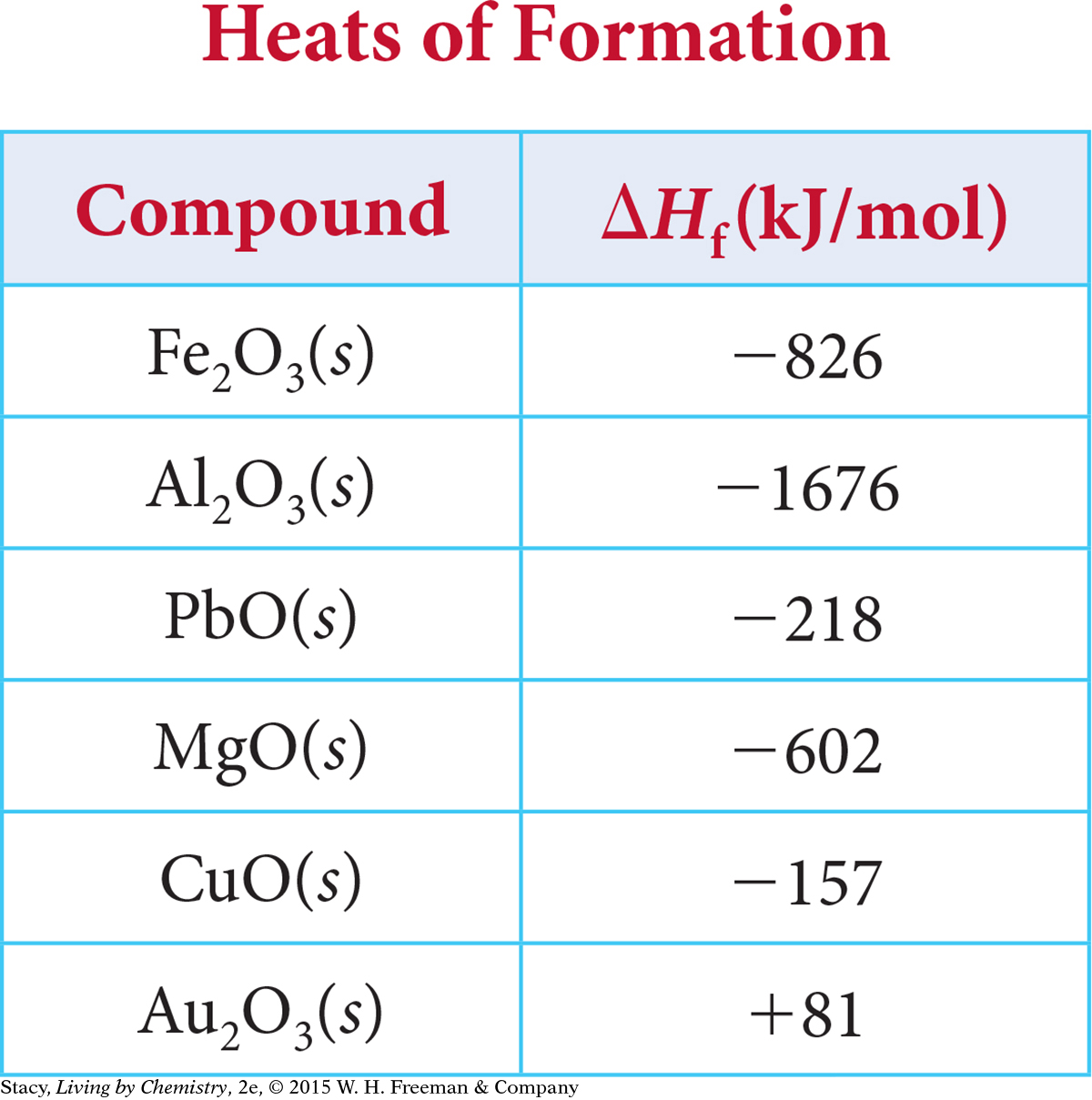
Example 1
Oxidation of Mg and Fe
Which reaction will release more energy?
2Mg(s) + O2(g) → 2MgO(s)
4Fe(s) + 3O2(g) → 2Fe2O3(s)
Solution
The heat of formation per mole of product can be found in the table above. Multiply ΔHf by the number of moles of each product to get the heat of the reaction, ΔH:
(–602 kJ/mol)(2 mol MgO) = –1204 kJ
(–826 kJ/mol)(2 mol Fe2O3) = –1652 kJ
The oxidation of iron, or the formation of iron (III) oxide, Fe2O3, produces more energy than the oxidation of magnesium. Heats of formation are not limited to metal oxides. For example, the heat of formation of magnesium chloride, MgCl2, is –2686 kJ/mol.
Reversing Oxidation
Reversing Oxidation
When an oxidation reaction for a metal is reversed, the resulting products are a pure metal and oxygen gas. This reaction is a decomposition reaction. The metal oxide decomposes and one of the products is a metal element. The decomposition of a metal oxide is one way to extract pure metals from metal compounds. But how difficult is it to accomplish energetically? Consider the decomposition of aluminum oxide, Al2O3:
2Al2O3(s) → 4Al(s) + 3O2(g)
556
Energy diagrams for the oxidation of aluminum and for the decomposition of aluminum oxide are shown here. Take a moment to examine the energy changes.
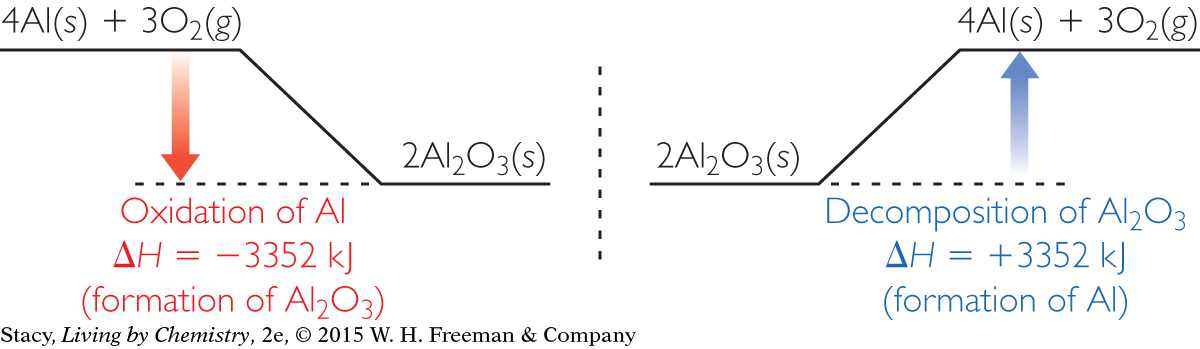
The oxidation of aluminum to form aluminum oxide is very exothermic. The energy for the reverse reaction is equal and opposite. So the decomposition of aluminum oxide is highly endothermic. This means that a large amount of energy is required to extract pure aluminum from aluminum oxide.
Indeed, aluminum was not discovered and brought into use until about 200 years ago, partly because so much energy is required to extract the pure aluminum metal from aluminum ores. The simple heating of aluminum ores does not provide enough energy to decompose the aluminum compounds and extract aluminum metal.
Example 2
Extracting Al
How much energy is required to extract a mole of aluminum metal from aluminum oxide, Al2O3?
Solution
The equation for the decomposition of aluminum oxide is
2Al2O3 → 4Al(s) + 3O2(g)
This process is the reverse of the formation of Al2O3(s), for which ΔHf = –1676 kJ/mol. (See the table on page 555.)
Because 2 mol of Al2O3(s) are decomposed in the equation, the heat of reaction, ΔH, is 2(1676 kJ/mol), or 3352 kJ/mol. Note that the sign is positive because the reaction is reversed.
Because 4 mol of aluminum are produced, the energy required to extract 1 mol of aluminum metal is one-fourth of the heat of reaction.
3352 kJ/mol/4 = 823 kJ/mol Al
THE HISTORY OF METAL USE

It is apparent from archeological sites that gold was in common use thousands of years before iron. This makes sense from the energy data for these metals. The metals that have been in use the longest are the ones that are easiest to extract from their compounds.
557
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
The process of removing metals from their ores is called smelting. It is usually necessary to add carbon to assist in the process of removing metals from ores. Smelting is often performed at high temperatures so that the metal obtained is already molten and ready to be cast.

This table shows the energy data for the decomposition of metal oxides to metals. To extract the metals, people had to construct furnaces that transferred a sufficient amount of heat.
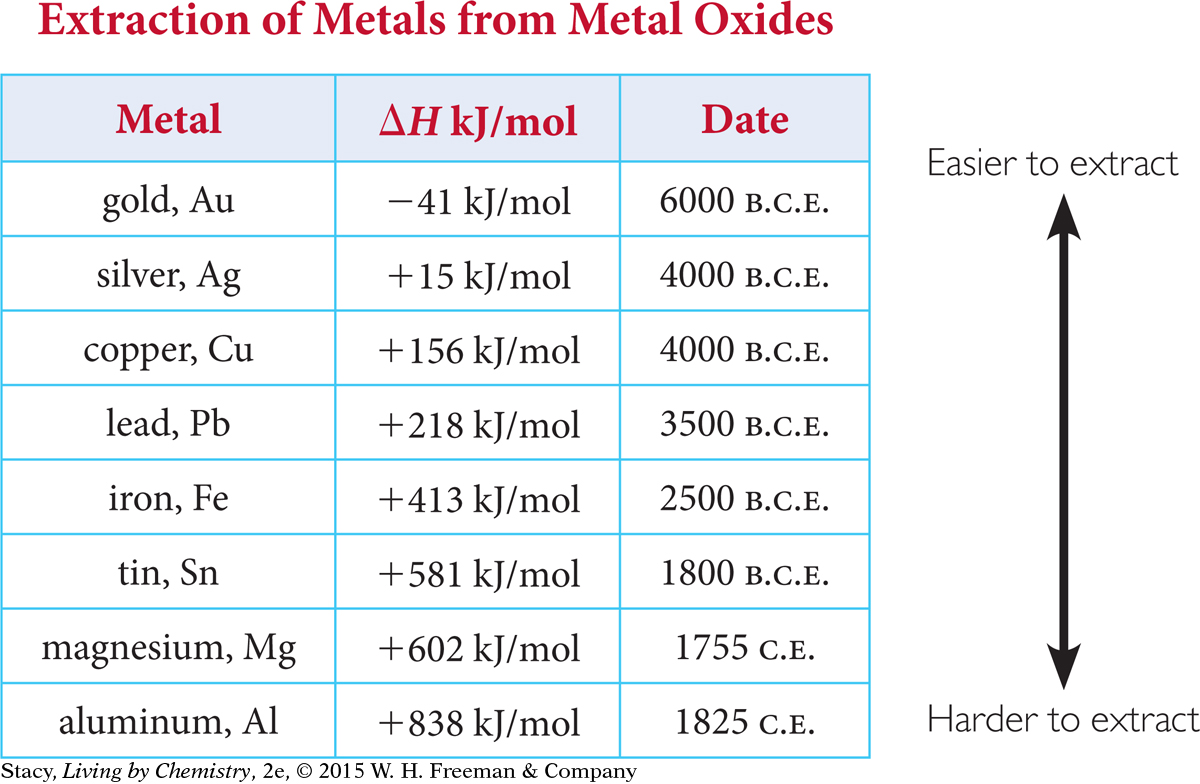
The difficulty of extraction of various metals from metal compounds is reflected in the chronological “discovery” and use of these metals through history. The more energy it takes to extract a metal, the later the metal was put into practical use.
Metals that are easily oxidized are hard to extract. In other words, the oxidation reaction is harder to reverse. These are also the metals that tarnish and rust easily. Gold is often found in its pure form in nature. This is because gold is not easily oxidized and is fairly unreactive.
LESSON SUMMARY
LESSON SUMMARY
How much energy is transferred during oxidation of metals?
KEY TERM
heat of formation
The formation of metal oxides is usually an exothermic reaction. This means oxidation reactions may be valuable sources of energy. The heat of formation of a compound is the thermal energy change associated with the formation of one mole of that compound from its constituent elements. The decomposition of metal oxides into pure metals and oxygen is the reverse of an oxidation reaction. These reactions are generally endothermic. This means it requires an input of energy to extract a metal from a metal compound. Metals that are easily oxidized (with high heats of formation) are more difficult to extract from their metal compounds than metals that are less easily oxidized (with low heats of formation).
Exercises
Reading Questions
Of all the possible reactions in this lesson, which ones have the potential of being the best sources of energy? Explain your answer.
What does the heat of formation indicate about a metal in terms of its practical use as a metal?
Reason and Apply
558
How much energy is released when 10 mol of lead (II) oxide, PbO, are formed?
Explain why metals that are easily oxidized are not found in nature in their pure forms. Name two of these metals.
Draw the energy diagrams for the oxidation of copper and its reverse reaction. Include the energy data.
How many kilojoules of energy does it take to extract 6 mol of tin from tin (II) oxide, SnO?
How many kilojoules of energy does it take to extract 10 g of copper from copper (II) oxide, CuO?
Which would be a source of more energy, the formation of 6 mol of magnesium oxide, MgO, or the formation of 2 mol of aluminum oxide, Al2O3? Explain.
Research how one of the metals of antiquity was extracted from metal compounds dug out of the Earth. Describe the process and draw a sketch.