LESSON 112: Current Events: Electrochemical Cell
THINK ABOUT IT
Batteries are used extensively in our daily lives. They are in our cell phones, automobiles, electronic toys, mobile music players, and wristwatches. A battery contains a controlled redox reaction.
How can you use a redox reaction as an energy source?
To answer this question, you will explore
Electrochemical Cells
Maximizing the Potential
Batteries
Electrochemical Cells
EXPLORING THE TOPIC
Electrochemical Cells
The combustion of gasoline powers cars. The combustion of methane is used to cook food and heat homes. But it would be hard to imagine a cell phone or a toy running off a combustion reaction.
There is another way, besides burning, to make use of the energy of a reaction. In the case of many oxidation-reduction reactions, the transfer of electrons can be converted into electrical energy using an apparatus called an electrochemical cell.
CONTROLLING REDOX REACTIONS
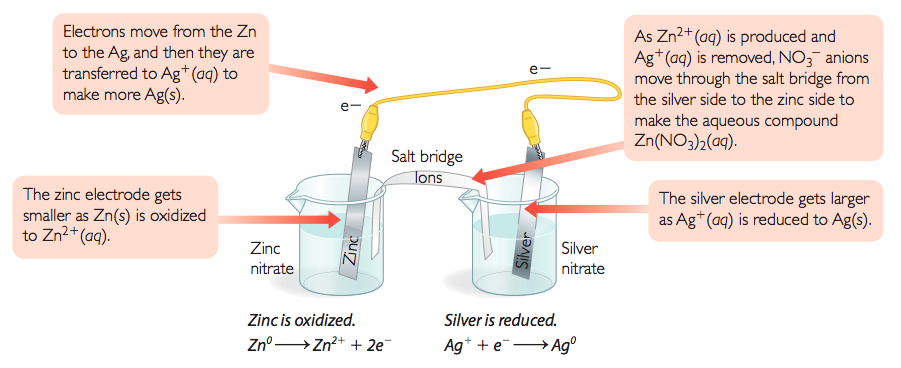
The first step to controlling a redox reaction is to separate the oxidation and reduction reactions. One possible way to do this is shown. Two strips of metal called electrodes are placed in two beakers. Both beakers are filled with aqueous salt solutions called electrolytes. Electrons move through a wire connecting the two metal strips, and ions move through a salt bridge connecting the two electrolyte solutions.
CONSUMER CONNECTION
CONSUMER
CONNECTION
The forward reaction of a car battery is used to start the engine, but once the engine is running, energy from the combustion of gasoline is transferred to mechanical energy and then converted to electrical energy by the alternator to reverse the reaction, recharging the battery. When you jump-start a car, you leave the engine running for a while to recharge the battery.

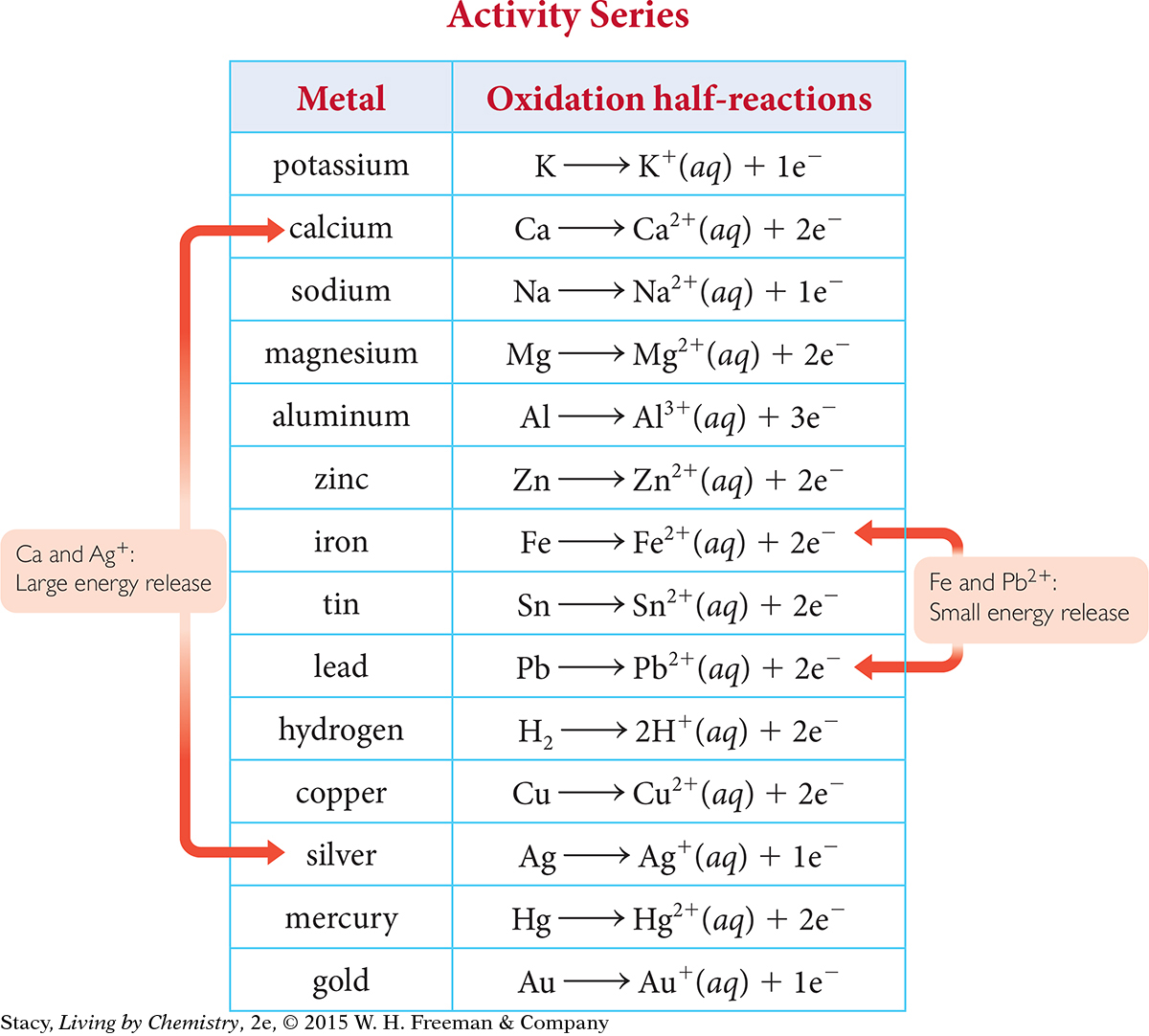
You can use the activity series table below to help you predict what will happen in the electrochemical cell. Because zinc is higher on the chart than silver, zinc is the more active metal and you can expect it to be oxidized. Likewise, because silver is not a very active metal, you can expect it to be reduced.
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
If you place Zn(s) and Ag+(aq) in the same beaker, the reaction will occur with direct transfer of electrons from the Zn(s) to the Ag+(aq). The reaction just goes until one or both reactants are used up.
Separating the reactants allows you to control the reaction and to create an electrical current. Each half of an electrochemical cell is called a half-cell. The reaction in the electrochemical cell can be stopped and started when desired. It will proceed only when the circuit is closed and the two halves of the reaction are connected. The reaction that takes place in each half-cell is called a half-reaction.
Maximizing the Potential
Maximizing the Potential
Not every combination of metals and metal salts results in the same output of energy. Generally, the farther away two metals are from each other on the activity series list, the more energy that will be produced by a reaction between them. For example, the energy released when calcium and silver are paired is greater than the energy released when iron and lead are paired.
Example 1
Magnesium-Tin Cell
Imagine you want to create an electrochemical cell using magnesium, Mg, and tin, Sn.
Which metal would be oxidized and which would be reduced?
What ion will form and what metal will be plated out of solution as a solid?
Write the half-reactions and the net ionic equation.
Solution
Magnesium is higher in the activity series.
Magnesium will be oxidized. Tin will be reduced.
Tin will be plated out onto the tin strip. Magnesium ions, Mg2+(aq), will form.
The half-reactions are Mg0 → Mg2+ + 2e– and Sn2+ + 2e– → Sn0. Adding these, the net reaction is Mg(s) + Sn2+(aq) → Mg2+(aq) + Sn(s).
The concentrations of the reactants, as well as the identities of the metals and metal ions used, can affect the amount of energy that is produced.
VOLTAGE
The energy that comes out of an electrochemical cell is expressed in volts. The voltage of a battery lets you know how much energy is “stored” in it, that is, how much potential it has to produce electricity.
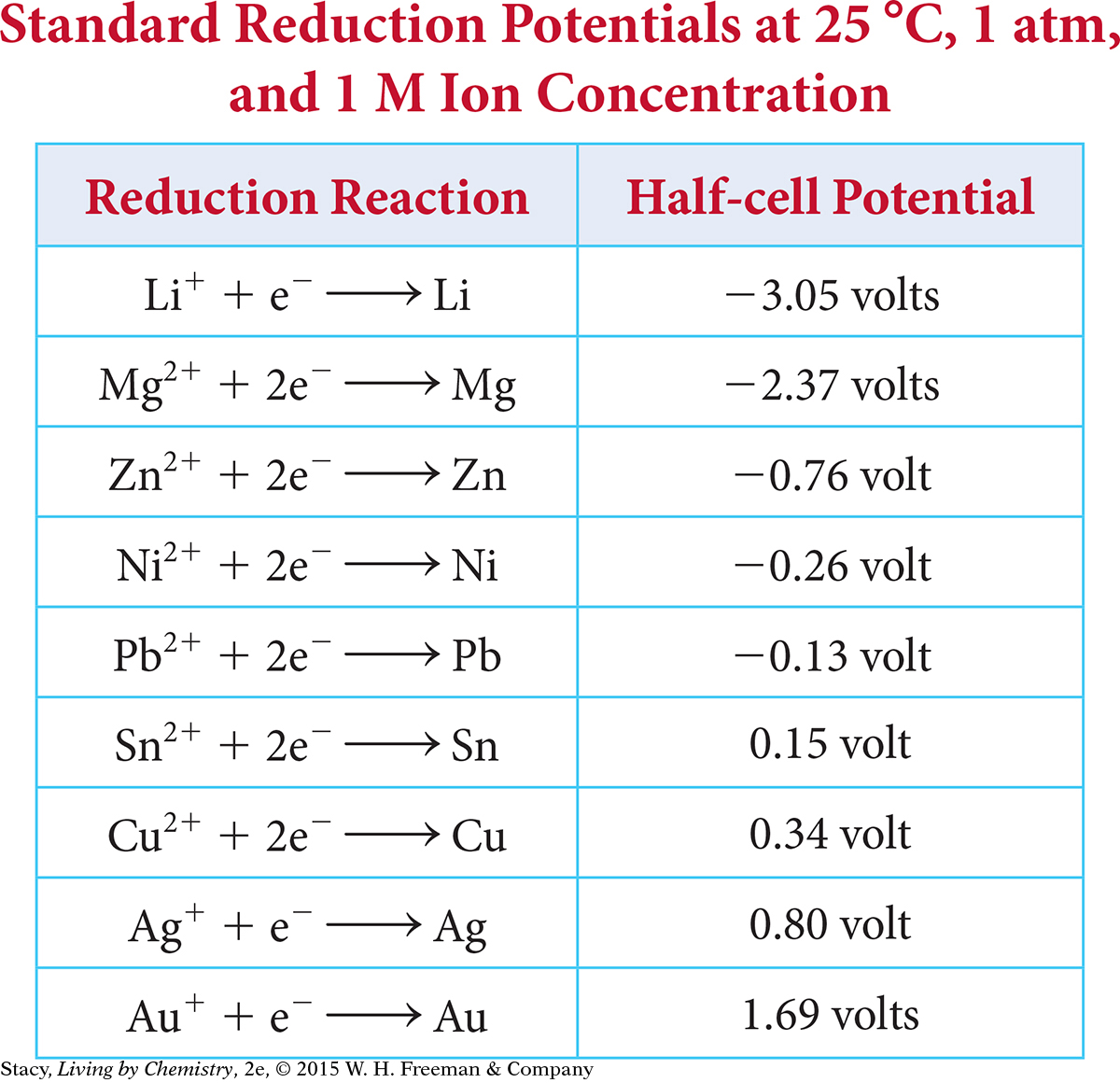
Chemists can predict the voltage of an electrochemical cell by examining the two half-reactions involved. The measurement of the voltage of half-cells takes place under uniform conditions. These voltages are normally expressed as reduction half-reactions and are listed in a standard reduction potentials table.
The overall voltage of an electrochemical cell can be calculated by finding the difference between the potentials of the half-reactions. This means subtracting the voltage of the oxidation process from the voltage of the reduction process.
Example 2
Silver-Zinc Electrochemical Cell
Determine the voltage of a silver-zinc electrochemical cell.
Solution
Write the half-reactions for silver and zinc from the standard reduction potentials table on page 571. The half-reaction that is more positive will proceed as a reduction, and the half-reaction that is more negative will proceed as an oxidation (in the reverse direction).
| This half-reaction proceeds as reduction. | Ag+ + e– → Ag | 0.80 volt |
| This half-reaction proceeds in the opposite direction as oxidation. | Zn2+ + 2e– → Zn | –0.76 volt |
Next, subtract the reduction potential of the oxidation half-reaction from the reduction potential of the reduction half-reaction.
0.80 – (–0.76) = 0.80 + 0.76 = +1.56 volts
This cell has a voltage of +1.56 volts.
Batteries
Batteries
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Batteries often contain substances that are potentially hazardous to living things. It is important to dispose of them properly so that they do not contaminate the water supply or the soil. This means they should be taken to a recycling center, not put in the trash.

Electrochemical cells have been developed into batteries, which are basically containers of chemicals just waiting to be converted to electrical energy. The chemical reaction within a battery does not take place until the two reactions are connected to one another. When you flip the switch on a flashlight, you are connecting the halves of the battery inside. If you forget and leave the flashlight on, the chemical reaction inside will continue until the battery is dead.
Some batteries are rechargeable. This means that once the battery has died, an electric current can be run through it in the opposite direction to push the reverse reaction to occur. This restores the original reactants, and the battery is good once again.
A battery that is not connected to anything still has a voltage, even though it is not yet doing any work. Most of the batteries in your household can produce voltages from about 1.25 volts to 9 volts.
LESSON SUMMARY
LESSON SUMMARY
How can you use a redox reaction as an energy source?
KEY TERMS
electrolyte
electrochemical cell
half-cell
half-reaction
voltage
You can convert the transfer of electrons that occurs during oxidation-reduction into electrical energy. By separating the reactants into half-cells, you can control and “store” electrical energy. The activity series allows you to choose electrodes and electrolytes that will produce electricity. The voltage of an electrochemical cell can be calculated from the half-cell potentials.
Exercises
Reading Questions
Explain how to determine the direction of electron transfer in an electrochemical cell.
Explain how you might choose which reactants to put together in an electrochemical cell.
Reason and Apply
Suppose that you make an electrochemical cell. One beaker has a calcium metal electrode and a solution of aqueous calcium ions, Ca2+(aq). The other beaker has an iron metal electrode and a solution of aqueous iron ions, Fe3+(aq).
Write the half-reactions for each beaker.
Use the activity series table on page 570 to decide which metal is oxidized and which metal cation is reduced.
Make a sketch of the electrochemical cell. Show the reaction that occurs in each beaker.
Write a net ionic equation for the reaction that takes place to generate electricity.
Repeat Exercise 3 with the pairs of metals here.
copper and tin
silver and aluminum
copper and lead
Predict which combination of metals in Exercises 3 and 4 will result in the greatest voltage. Explain your prediction. Then calculate the voltages of the redox reactions. Was your prediction correct?
What are the pros and cons of using combustion reactions as a source of energy?
What are the pros and cons of using electrochemistry as a source of energy?
Research common batteries, such as AA or D cell batteries, to find out the chemistry behind them. List the metals and compounds commonly used in their construction. Car batteries are usually 12-volt. Research car batteries and describe how such a high voltage is achieved. When car lights are left on for a long time, this can cause a “dead” battery, which will require a jump-start from another car battery. In chemical terms, what does it mean to have a “dead” car battery? What does “jump-starting” a car battery mean in terms of the overall redox reaction? Research and report on your findings.