LESSON 116: How Absorbing: Spectroscopy
THINK ABOUT IT
While enjoying a day at the beach, you notice a person whose skin has turned quite red. In contrast, your skin looks the same as it did in the morning when you left your house. This is because you have applied sunscreen several times during the day. The claim on the package is that sunscreen protects against harmful UV radiation. Although you cannot see UV radiation, how is it possible to determine which substances block UV radiation effectively?
How is it possible to measure electromagnetic radiation that you cannot see?
To answer this question, you will explore
Light Detectors
Spectroscopy: Studying Matter with Light
Light Detectors
EXPLORING THE TOPIC
Light Detectors
A light detector is a device that can sense electromagnetic radiation. The detector provides some type of signal when it comes into contact with electromagnetic radiation. Some detectors, such as solar cells, produce an electrical signal that converts visible light into electricity. Radio antennas detect radio waves. The radio then converts the signal detected by the antenna to sound. Your skin signals exposure to UV radiation by turning red. In this lesson, you will explore what happens when paper coated with certain chemical mixtures responds to electromagnetic radiation by changing color.

Solar cells detect visible light.
pvicens/Getty Images
|

The radio detects radio waves.
maxim ibragimov/Shutterstock
|
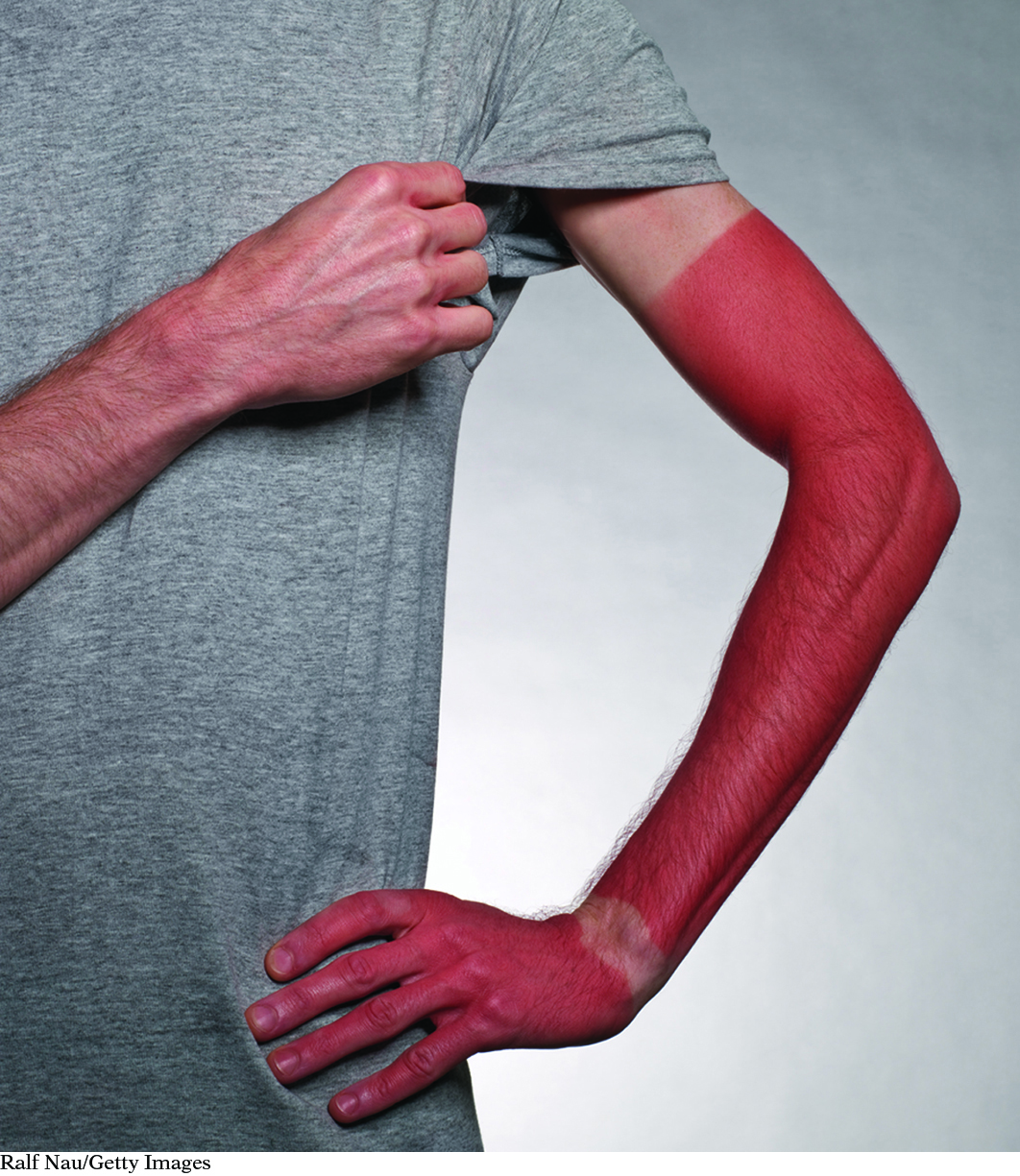
Your skin detects UV radiation.
Ralf Nau/Getty Images
|
UV-SENSITIVE PAPER
For more than 150 years, people have been creating images and photos by using light-sensitive paper and film to capture light. One way to create an image this way is to place an object that blocks the incoming electromagnetic radiation onto the light-sensitive paper. The electromagnetic radiation only strikes the paper where it is not blocked by the object. This creates a silhouette of the object.

Some UV-sensitive paper is coated with a mixture of compounds. Before exposure to UV radiation, the mixture of compounds is pale green. This color indicates that one of the compounds in the mixture absorbs all colors except for green. Green light is reflected.
The green reactant compounds are soluble in water. If the paper is submerged in water, the green compounds dissolve and wash away. With the green reactants removed, the paper is no longer UV-sensitive. If you expose it to UV radiation, it will not turn blue. Instead, it will remain white.

If you shine UV radiation on the green UV-sensitive paper, the energy that is transferred provides the energy needed to cause a chemical reaction to occur. The reactant that causes the green color changes into a new compound that is blue. The paper is blue because the new compound reflects blue light.
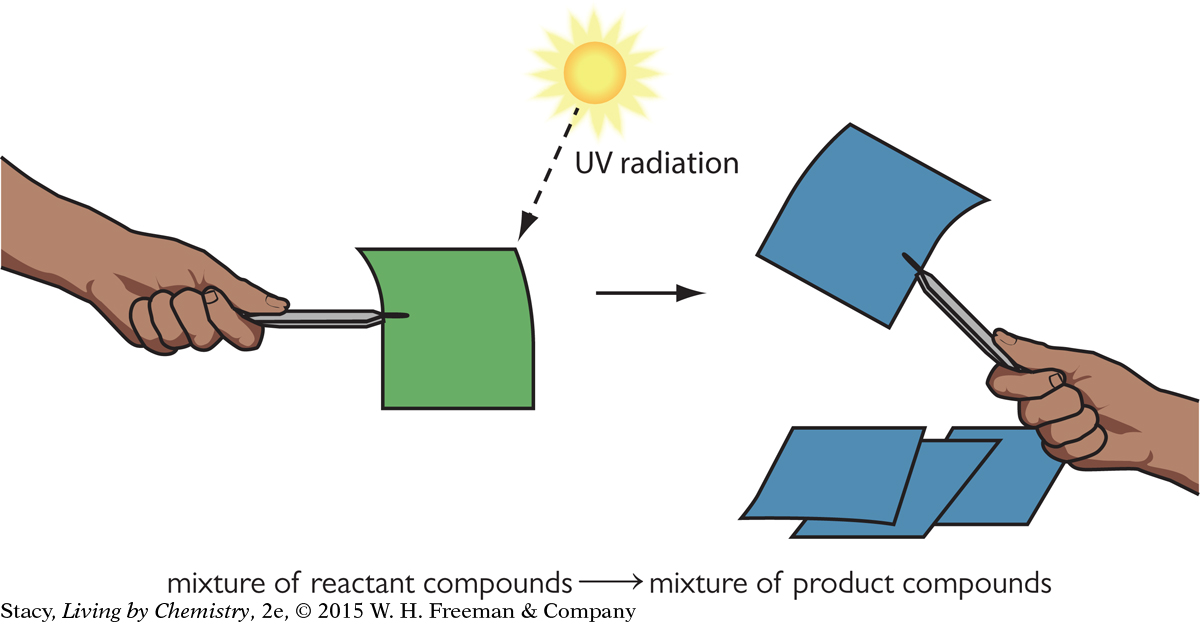
The longer the paper is exposed to UV radiation, the deeper the shade of blue it turns. The reaction will continue until all the reactants are used up and the paper has the deepest blue color. The amount of the green compound is a limiting reactant. So one way to stop the reaction is to expose the paper to UV radiation until all limiting reactant is used up in the reaction.
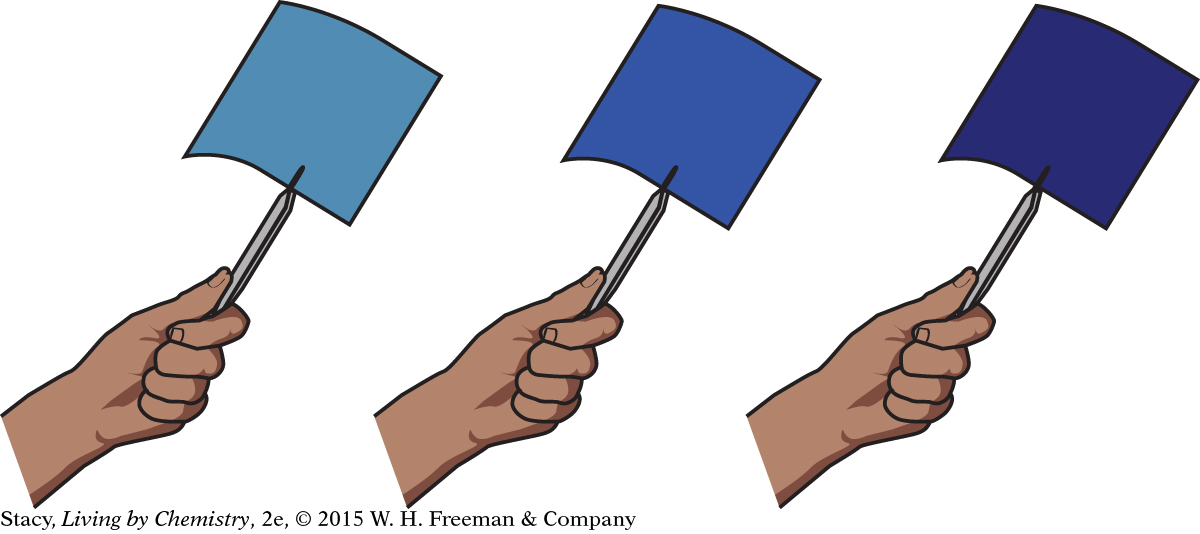
Another way you could stop the reaction is related to the difference in solubility between the green reactant compound and blue product compound. The green reactant compound is soluble in water, but the blue product compound is insoluble in water. If you expose the paper to UV radiation for a very short time, only some of the reactant compound will turn blue. The paper will appear to be a pale blue color and is a mixture of green reactant compound that has not yet reacted and blue product compound. If you then submerge the partially reacted paper in water, the green reactant compound will dissolve in the water and can be washed away. The blue product compound remains attached to the paper because it does not dissolve in the water. Now, even if you were to expose the partially reacted and washed paper to UV radiation again, it would no longer change to a deeper blue. It would remain the same pale blue color because there would no longer be any green reactant compound available to change to blue product compound.
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
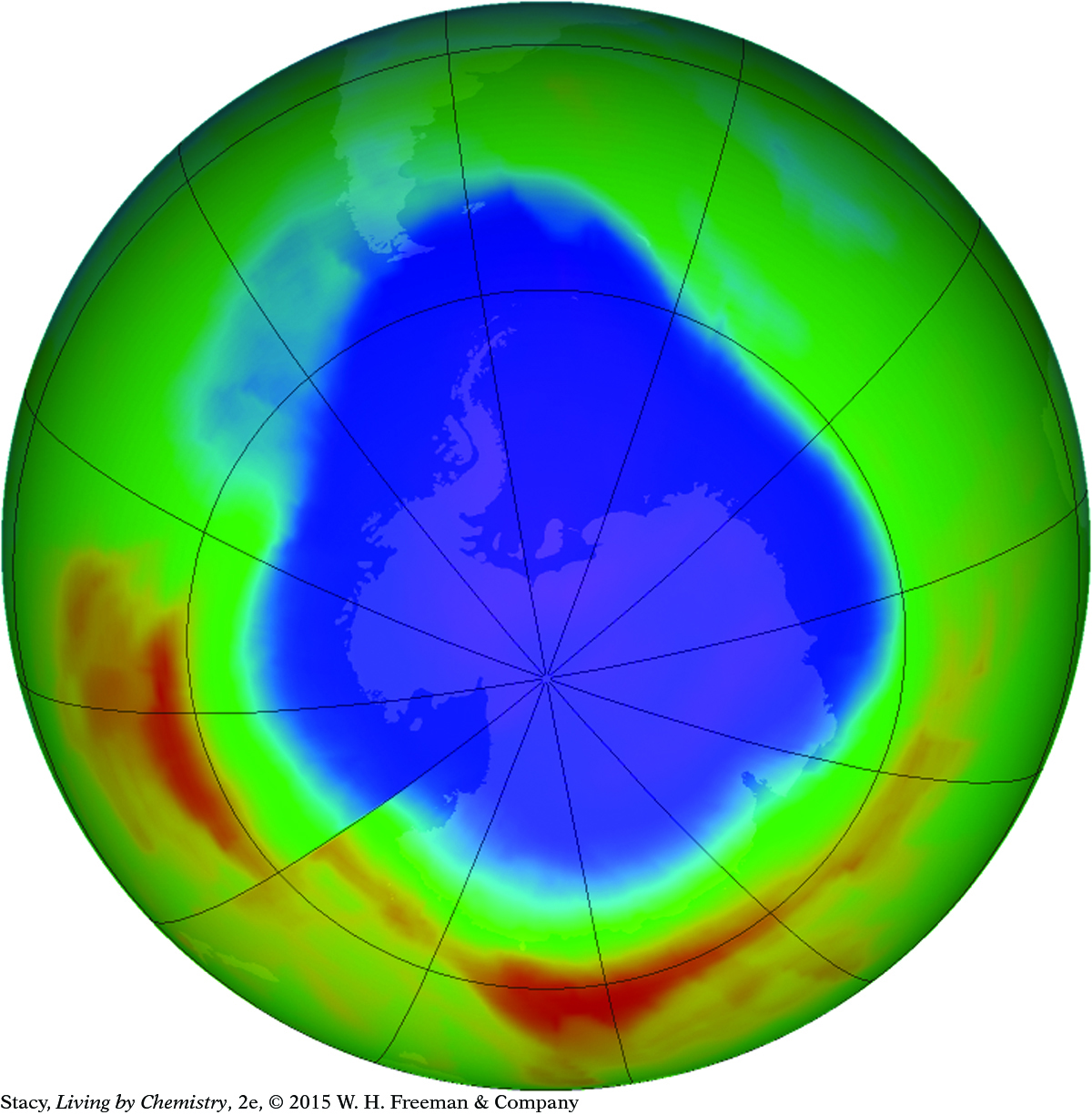
Ozone in the upper atmosphere protects us against UV light, which can cause serious damage to eyes and skin over time. Thinning of the ozone layer over Antarctica is caused by chemical reactions in which pollutants break apart ozone molecules. International cooperation to control pollutants has led to less stress on the ozone layer and gradual improvement. The expanse of blue here is the “ozone hole”:
CREATING AN IMAGE
Now imagine that you place a small object on top of the UV-sensitive paper and expose the paper and object to UV radiation. The paper turns blue in the areas surrounding the object. But underneath the object, the paper remains green. This is because the object blocks UV radiation, preventing it from shining on the paper directly beneath the object. There is a green image of the object on a blue background after exposure to UV radiation.
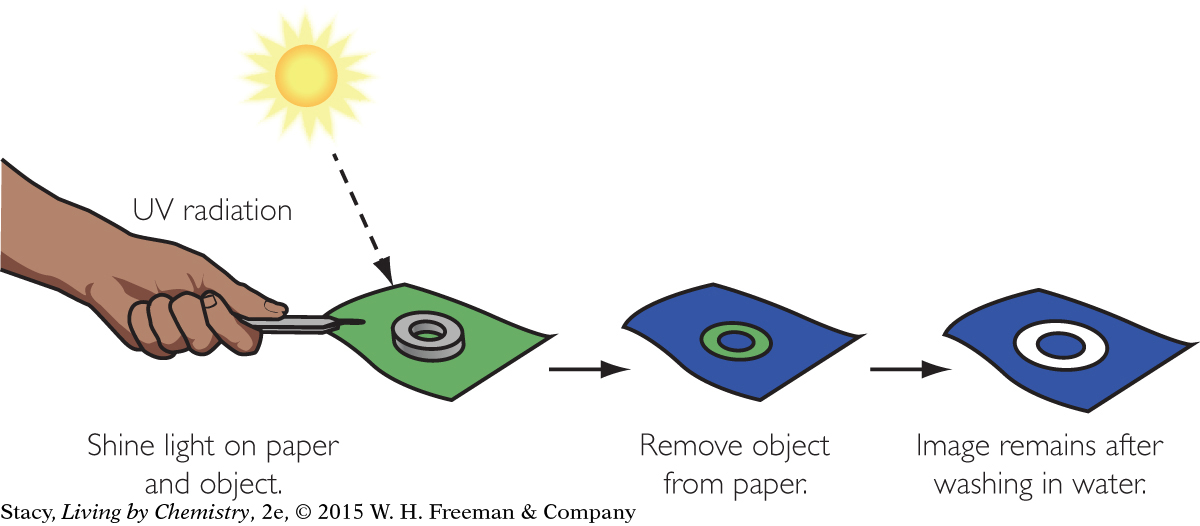
If the paper with the green image is exposed to light, the green compounds will react and the paper will be completely blue. However, if you protect the paper with the green image from UV radiation and then wash away the green compounds, a white image of the object will remain permanently.
Spectroscopy: Studying Matter with Light
Spectroscopy: Studying Matter with Light
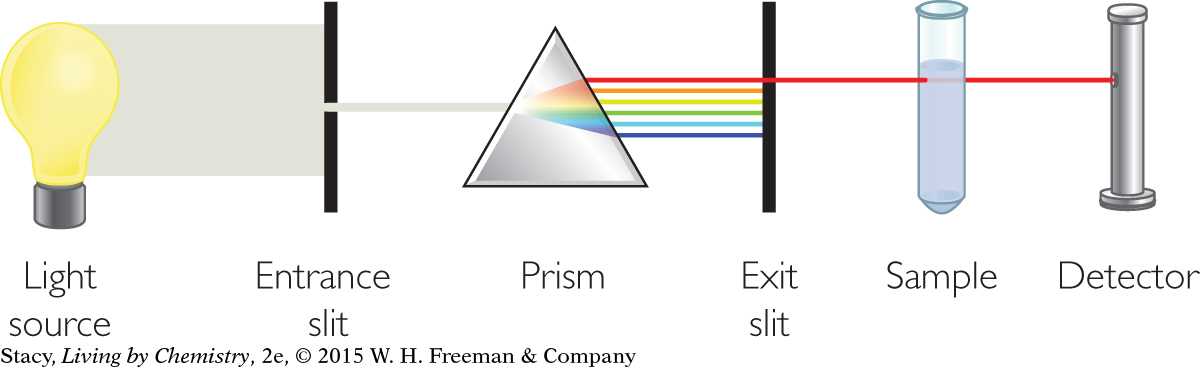
Scientists make use of light detectors to create instruments called spectrometers to study properties of matter. A spectrometer has four main components: a source of electromagnetic radiation, a device, such as a prism, to separate the wavelengths of light, a sample holder, and a light detector. By using electromagnetic radiation of different wavelengths, it is possible to gain a lot of information about atoms, molecules, and compounds. It is also possible to learn about the concentration of a substance and its identity depending on the choice of electromagnetic radiation. These methods for analyzing compounds are referred to as spectroscopy.
STUDYING WHAT YOU CANNOT SEE
Some substances, such as sunscreen, clothing, glass, and plastic, absorb UV radiation. Other substances, such as the atmosphere, transmit some UV radiation. You can use UV-sensitive paper as a simple UV detector to see how a substance behaves when exposed to UV light. Although it’s not a spectrometer, this tool is similar to one in some ways. It allows you to study what you can’t see. The light source for this tool is UV radiation, the sample is the substance you test, and the detector is the UV-sensitive paper.
MEDICAL CONNECTION
MEDICAL
CONNECTION
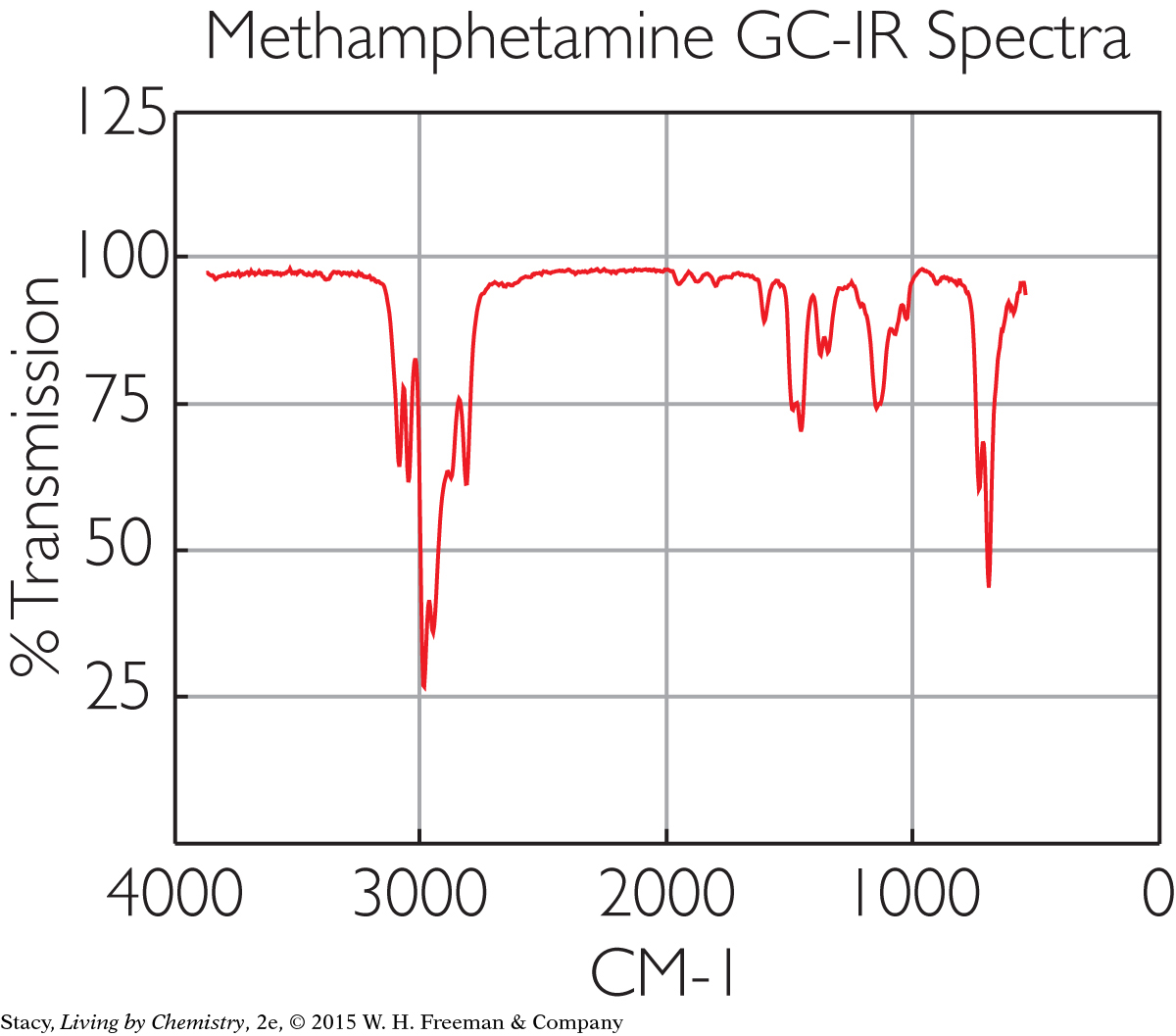
Spectroscopy can be used to identify substances, such as medicines or illegal drugs that a person has ingested. Infrared spectroscopy produces a reading that shows the molecules in an unknown substance. The absorption of infrared light indicates which molecules are present and in what proportions. Shown here is the infrared spectroscopy “fingerprint” of the chemical methamphetamine.
First, you have to place your sample substance on a piece of UV-sensitive paper. It is best to spread the sunscreen on a thin piece of glass or plastic. Next, expose the sample on the paper to a source of UV radiation. If the substance absorbs UV radiation, the paper under the substance will remain green when exposed to the UV radiation source. If the substance transmits UV radiation, the paper under the substance will turn blue when exposed to the UV light source.
You can also use this setup to determine how different amounts of a substance block or transmit UV radiation. To do this, you need to examine samples with different concentrations of the substance being tested. For example, you might test sunscreens with the same active ingredient but different sun protection factor (SPF) ratings to see which ones block the most UV radiation. The SPF rating indicates the ability of the sunscreen to block UV radiation. Sunscreens with higher SPF ratings are more concentrated in the substance providing protection.
You could also use this tool to examine various light sources to compare the amount of UV radiation they emit. For example, the Sun emits a lot of UV radiation. In contrast, indoor lighting emits much less UV radiation. This is why you can get a sunburn when you are outside and exposed to the Sun but do not get a sunburn from indoor light sources.
Big Idea
Big Idea
Spectroscopy is a method of using electromagnetic radiation to study properties of atoms, molecules, and compounds.
LESSON SUMMARY
LESSON SUMMARY
How is it possible to measure electromagnetic radiation that you cannot see?
KEY TERMS
light detector
spectrometer
spectroscopy
Various sensors are used to detect electromagnetic radiation. Human eyes detect visible light as does the detector in a digital camera. It is also possible to use certain chemicals as light detectors that respond to different wavelengths of electromagnetic radiation. For example, UV-sensitive paper is coated with a chemical that changes color upon exposure to UV light. Longer exposures result in a color that is a deeper shade. This allows you to examine the amount of UV radiation emitted from various light sources, and to determine the ability of various substances to block UV radiation.
Exercises
Reading Questions
Why does UV radiation change the color of UV-sensitive paper?
Make a sketch of a spectrometer and label the main parts.
Homework
What happens if you leave a piece of UV-sensitive paper out in the Sun?
List three materials that block UV radiation.
What happens if you leave a piece of UV-sensitive paper out in a room with a dim red light?
Imagine that you want to measure two red solutions to determine if they have the same concentration of red dye or not.
What type of light source do you need for the spectrometer?
Sketch the placement of the light source, the detector, and the sample. Show the path of the light.
What do you predict you will observe if the two solutions are different?
Can you use UV-sensitive paper for the detector? Why or why not?
What differences do you predict if you use UV-sensitive paper to test sunglasses with UV protection compared with regular reading glass without UV protection?
Write a lab report for the experiment that you designed in class. In your report, include the title of the experiment, purpose, procedure, observations and conclusions.
