LESSON 119: How Dynamic
THINK ABOUT IT
A tightrope walker moves carefully and purposefully, keeping his balance as he goes. He tips back and forth while he walks, sometimes using an outstretched arm or a leg to counterbalance any movement one way or the other. He is maintaining his balance but not keeping still. Chemical systems that are at equilibrium maintain a similar type of dynamic balance.

What is chemical equilibrium?
To answer this question, you will explore
Models of Equilibrium
Chemical Equilibrium
Models of Equilibrium
EXPLORING THE TOPIC
Models of Equilibrium
If you put a small amount of sodium chloride, NaCl(s), in water, the process goes toward forming Na+(aq) and Cl−(aq) ion products. If you keep adding more and more NaCl(s), the solution will become saturated. Once this happens, no more NaCl(s) will dissolve. The excess NaCl(s) remains on the bottom of the container.
THE VIEW AT AN ATOMIC SCALE
To gain a better understanding of why there is a limit to the amount of NaCl(s) that dissolves in a given quantity of water, it is useful to consider what is happening at the atomic scale. The figure on the next page shows a model of how water molecules bump into the surface of the solid and carry the ions into the solution. This is due to ion-dipole intermolecular attractions between the Na+(aq) and Cl−(aq) ions and the water dipoles.
It is also possible to carry out the reverse process by evaporating the water. As the water evaporates, there is less space for the Na+(aq) and Cl−(aq) ions to spread out, and they bump into each other more often. The forces of attraction between ions cause the salt to re-form.
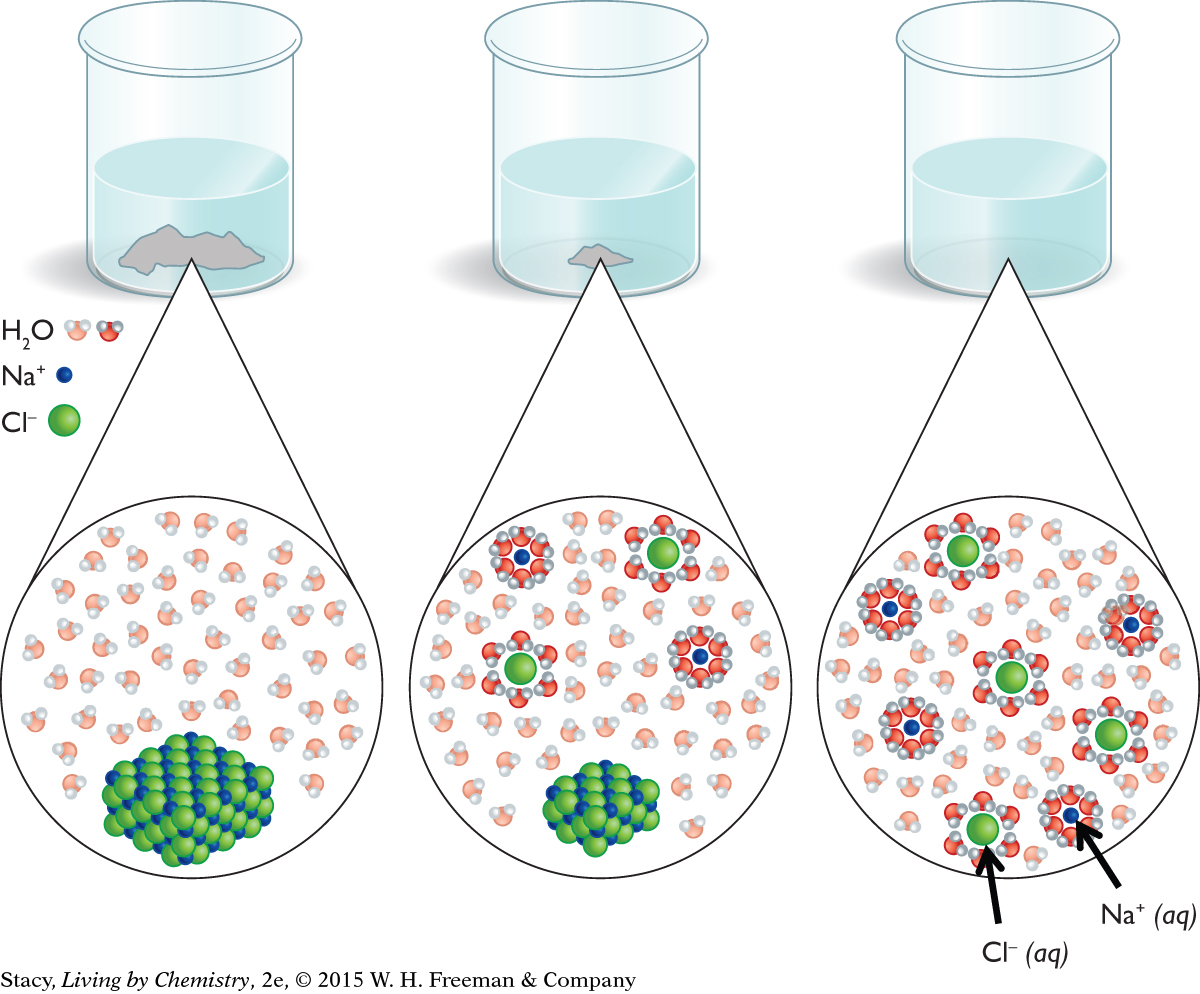
A DYNAMIC BALANCE
In a dilute aqueous solution of NaCl, the process of dissolving has gone entirely to the Na+(aq) and Cl−(aq) ion products. The ions do bump into one another, but enough of them do not cluster together to form a solid. For dilute solutions, you can represent the process of dissolving NaCl(s) by using a chemical equation with a single arrow in the direction of forming Na+(aq) and Cl−(aq) ions.

Ken Karp Photography
|

Ken Karp Photography
|
When the concentration of Na+(aq) and Cl−(aq) ions is large enough, solid salt is visible at the bottom of the container. The solution is referred to as saturated. For an observer, it appears that nothing is happening. However, on an atomic scale, the dissolving and precipitation processes are still happening. When some Na+(aq) and Cl−(aq) ions precipitate, there is enough room for more NaCl(s) to dissolve, and so on, back and forth. This explains why all the salt does not dissolve: some keeps precipitating. The saturated solution with excess solid NaCl is represented by a chemical equation with a double arrow to show that both the forward and reverse processes are occurring.
The saturated NaCl solution with excess solid on the bottom is at chemical equilibrium. Equilibrium is a dynamic state in which opposing processes occur at the same time. At equilibrium, the rate of the forward process is equal to the rate of the reverse process. Because these rates are equal, no change is visible.
In contrast, in the case of a dilute solution, the rate of the reverse process (precipitation) is never equal to the rate of the forward process (dissolution). In this case, all of the solid dissolves.
Big Idea
Big Idea
At equilibrium, the rate of the forward process is equal to the rate of the reverse process.
Example
Evaporation of Water

Consider the evaporation of water at 25° C.
Right after a rainfall, there are water puddles on the sidewalk. Once the rain stops and the sun is shining, the water puddles evaporate completely. Explain why. Provide a chemical equation to describe the process.
The water in the bottle shown in the picture does not evaporate completely. Explain why. Provide a chemical equation to describe the process.
How does chemical equilibrium explain the observation of droplets of water on the sides of the bottle above the water level?
Solution
The puddles evaporate and the gaseous water molecules spread out in the atmosphere. The reverse process (condensation) does not happen because the rate at which molecules attract one another is too slow to form water droplets. A chemical equation with a single arrow describes the process.
H2O(l) → H2O(g)
The water in the bottle does not evaporate completely because the closed container limits the space in which the water molecules can travel. When there are enough gaseous water molecules in the small space above the liquid water, the molecules bump into each other frequently enough to condense. This means that while water continues to evaporate, the gaseous water molecules are also condensing. So, the forward and reverse processes of evaporation and condensation are occurring simultaneously. A chemical equation with a double arrow describes the process.
H2O(l) ⇋ H2O(g)
The water in the bottle is at equilibrium. This means that the rate of evaporation is equal to the rate of condensation. When the gaseous water condenses, it can form droplets on the side of the bottle above the water level, or simply fall onto the surface of the liquid water.
Chemical Equilibrium
Chemical Equilibrium
You can use a paper-clip model to describe an irreversible process and a reversible process. The paper-clip model is a general example of any process involving an AB pair that separates into A and B.
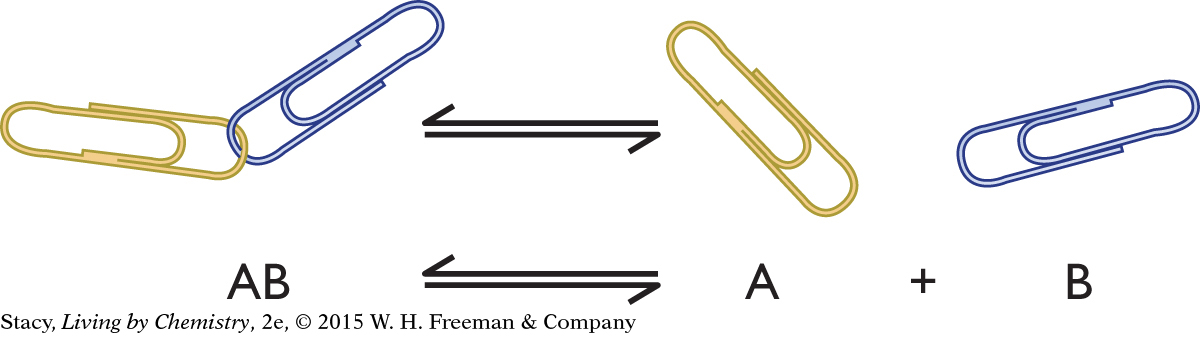
The paper-clip pair, AB, might represent:
a salt such as NaCl, which dissociates to Na+(aq) + Cl−(aq)
a strong acid such as HCl, which dissociates to H+(aq) + Cl−(aq)
an acid-base indicator, HIn, which dissociates to H+(aq) + In−(aq)
a molecule bound to a receptor, which separates into a molecule and the empty receptor
a molecule such as N2O4, which breaks apart into two molecules of NO2
It is useful to examine the features of the model for both irreversible and reversible processes.
IRREVERSIBLE VERSUS REVERSIBLE PROCESSES
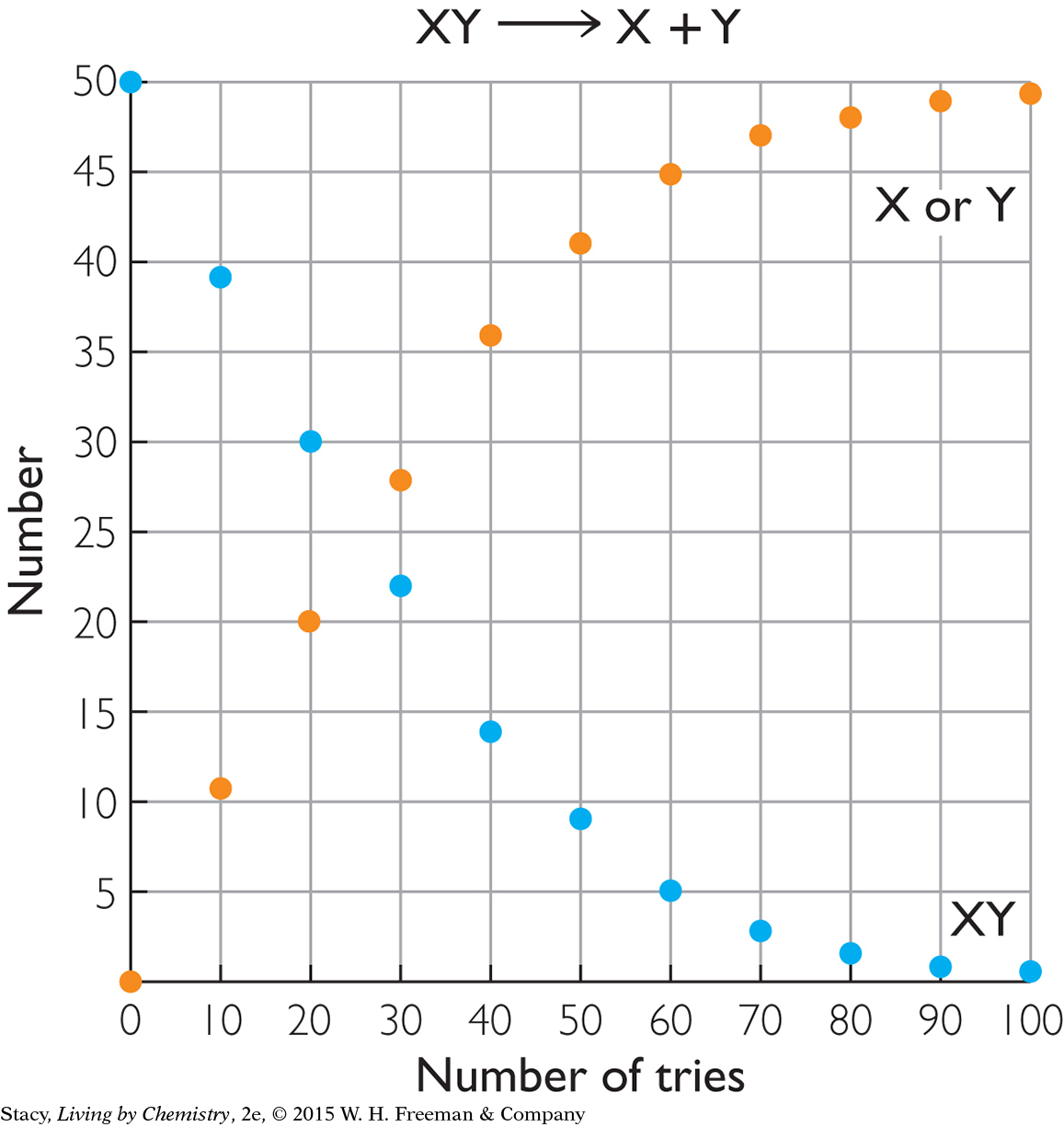
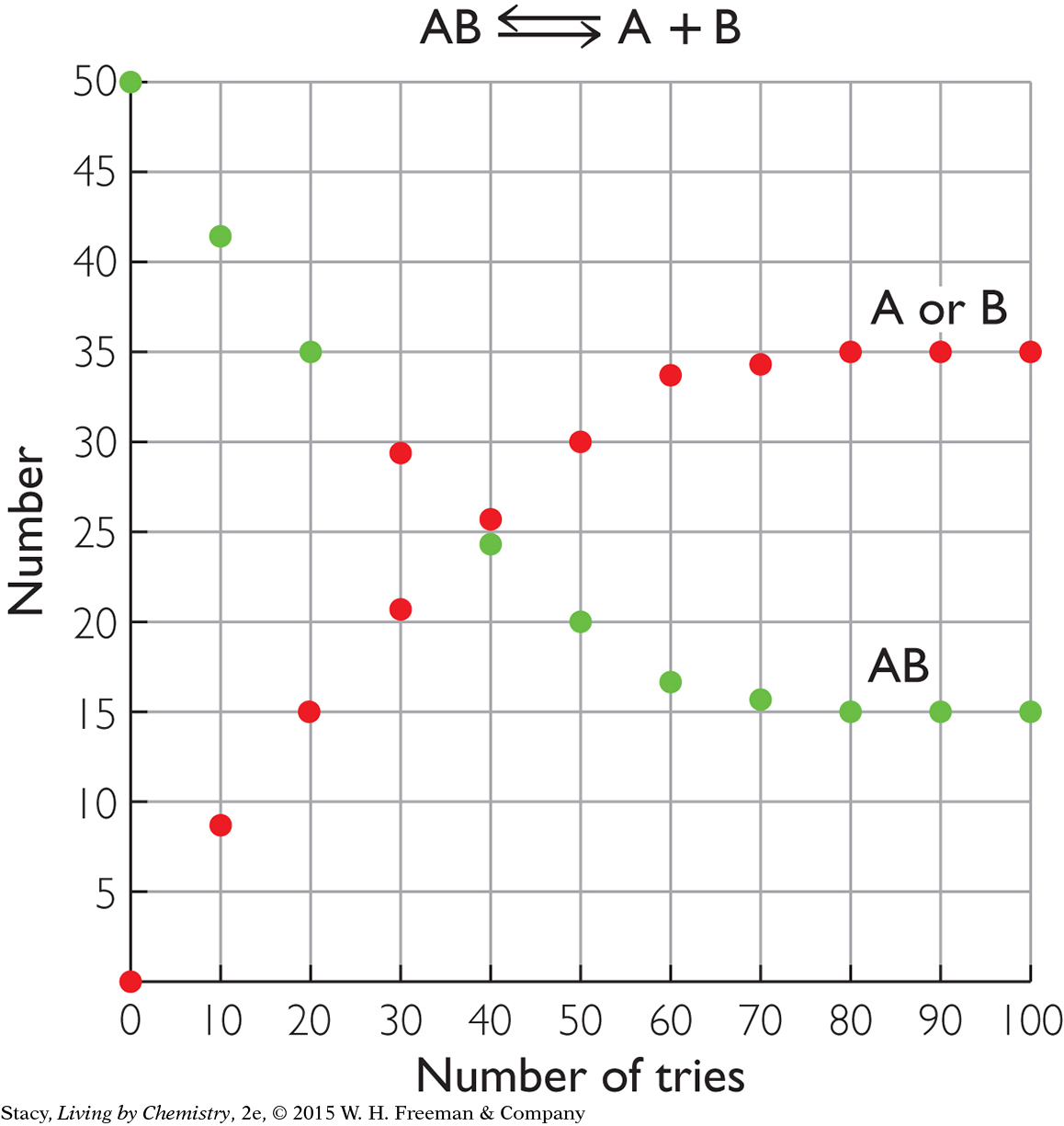
The two graphs below show what happens for two different starting materials, one labeled XY and the second labeled AB. Imagine that the first graph represents a collection of chocolate pieces, XY, with each piece broken into two smaller pieces, X and Y. The second graph might represent a collection of paper-clip pairs, AB, of two different colors that are pulled apart into single paper clips, A and B, and put back together again.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The word equilibrium also refers to the sense of balance that keeps people and animals from falling over. This sense of equilibrium is a result of combined input from the eyes, inner ears, pressure sensors on the skin, and the cerebellum in the brain. When your sense of equilibrium is impaired, you can experience motion sickness.

Take a moment to examine the graphs and describe the similarities and differences.
|
|
Here are some similarities that you might notice:
In both cases, the starting substance is breaking apart into two pieces.
At the start of the process, there are 50 XY pairs and 50 AB pairs.
The number of XY pairs decreases as the number of X or Y pieces increases. The number of AB pairs decreases as the number of A or B pieces increases.
Here are some differences that you might notice:
The number of XY pairs goes to 0 over time. Once you break the chocolate, you cannot put it back together again. The process is irreversible.
The number of AB pairs does not go to 0. There are 15 AB pairs remaining, even after a long time. This is because the reverse reaction makes new AB pairs. At equilibrium, there is a mixture of 15 AB pairs, 35 individual A pieces, and 35 individual B pieces.
If you examine the graph on the right, you might notice that the amounts of AB, A, and B in the mixture no longer change after about 80 tries. This indicates that equilibrium is reached: the amounts remain fixed.
Example
Strong versus Weak Acid
Consider the observations reported here for two aqueous solutions: one with hydrochloric acid, HCl, and a second with formic acid, HCOOH.
The process of dissolving hydrochloric acid, HCl, in water results in a solution of H+(aq) and Cl−(aq). If there are any HCl molecules remaining, the numbers are so tiny that they are not detected. Explain why. Base your explanation on the rates of the forward and reverse processes.
The process of dissolving formic acid, HCOOH, in water results in a solution of HCOOH, H+(aq), and HCOO−(aq). There are many HCOOH molecules in the solution compared with ion products. Explain why. Base your explanation on the rates of the forward and reverse processes.
Solution
Only the forward process occurs at a high rate. Once H+(aq) and Cl−(aq) form, they do not stay together long enough to produce a measurable amount of HCl(aq). (See the graph for XY.) So nearly all of the HCl is converted to products. This process is often represented with a single arrow. Because very nearly all the HCl molecules dissociate, HCl is called a strong acid.
HCl(aq) → H+(aq) + Cl−(aq)
The process does not go to completion to become products because the reverse process also occurs at a high rate. Once H+(aq) and HCOO−(aq) form, they do go back together again to make HCOOH. (See the graph for AB.) At first, the rate of the forward reaction is fast and the rate of the reverse reaction is slow. The rate of the forward reaction slows down as more HCOOH molecules dissociate. The rate of the reverse reactions speeds up as the number of H+ and HCOO− ions increases. Over time, the rates become equal and the process reaches equilibrium. At equilibrium, the amounts of HCOOH(aq), H+(aq), and HCOO−(aq) no longer change. This reversible process is represented with a double arrow. Because very few of the HCOOH molecules dissociate, HCOOH is called a weak acid.
HCOOH(aq) ⇋ H+(aq) + HCOO−(aq)
STARTING WITH ALL PRODUCTS
The two graphs below compare the same reversible processes but for different starting amounts. In the graph on the left, there are 50 AB pairs at the start but no A or B pieces. In the graph on the right, there are 50 A and 50 B pieces at the start but with no AB pairs. Take a moment to examine the graphs. What do you notice about the amounts at equilibrium?
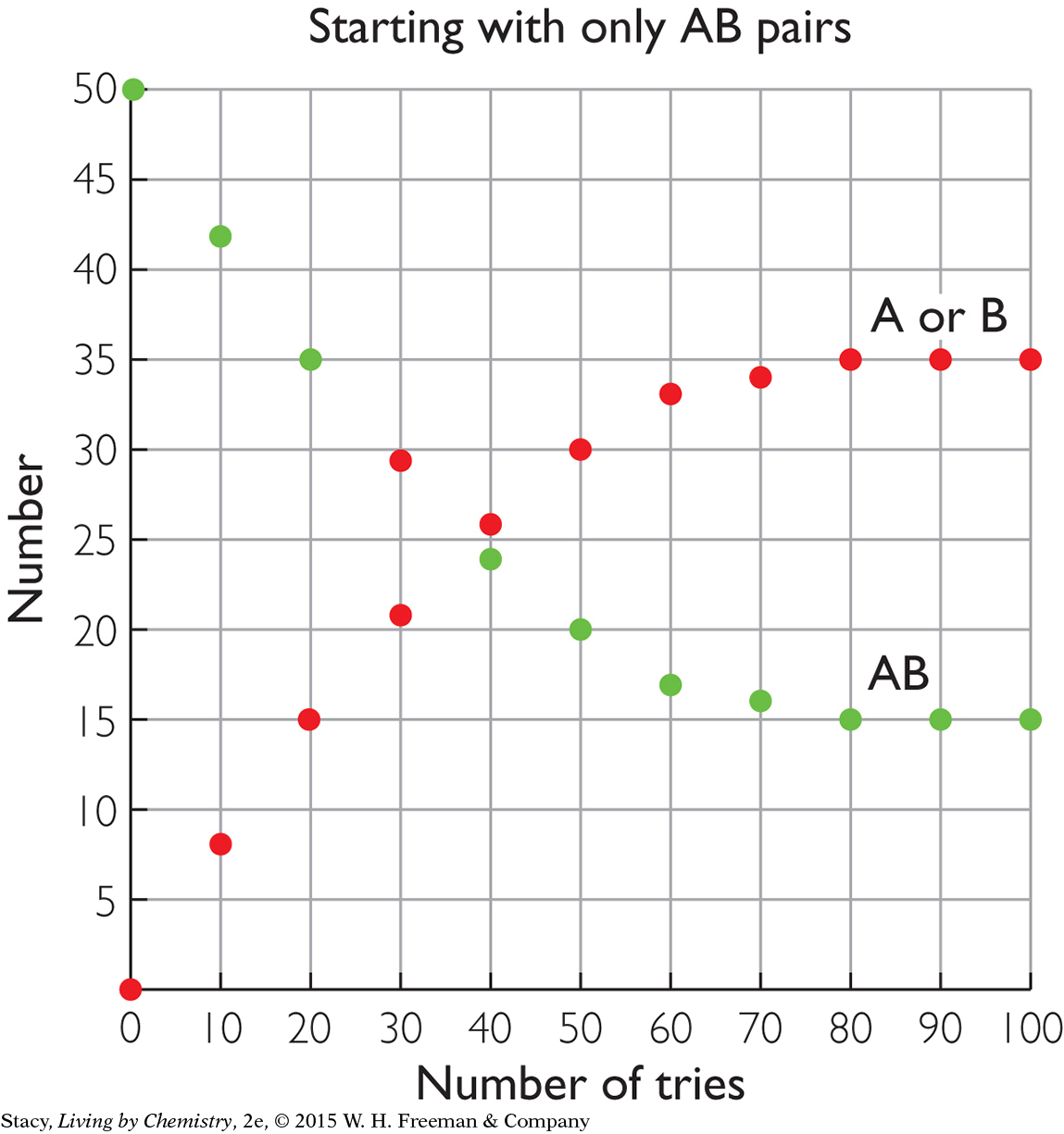
|
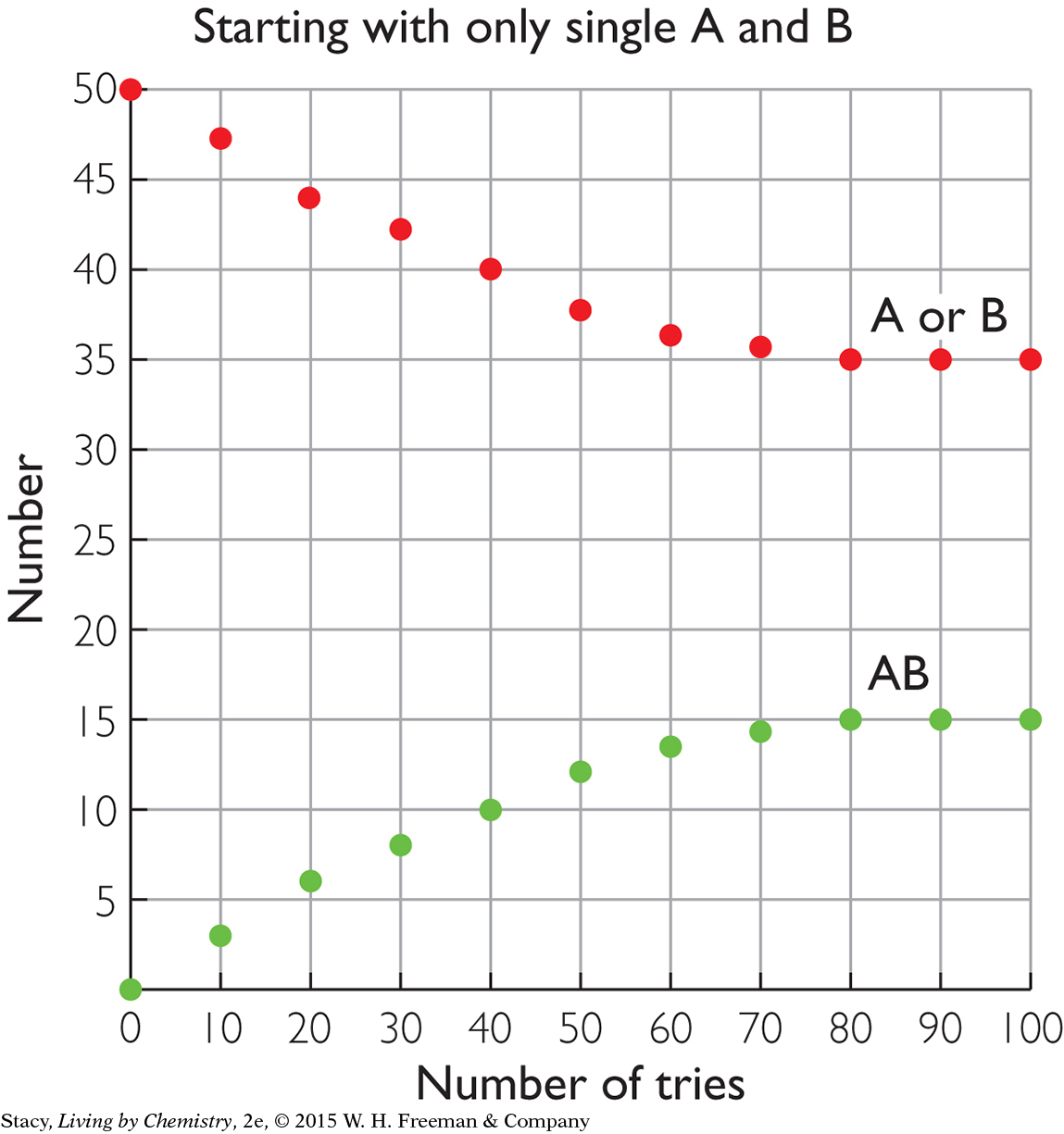
|
Important to Know
Chemical equilibrium means equal rates of forward and reverse processes, not equal amounts of reactants and products. The amounts remain fixed at equilibrium, but they are not necessarily equal.
After 100 tries, the amounts of AB, A, and B are the same in both cases. This indicates that equilibrium is a property of the mixture. It does not matter if you start with the substances on the left or right of the double arrow; the resulting mixture is the same.
LESSON SUMMARY
LESSON SUMMARY
What is chemical equilibrium?
KEY TERM
chemical equilibrium
Equilibrium is a dynamic process. The word dynamic means that things are constantly changing. Equilibrium is a balance that is reached between the forward and reverse processes in a reversible change. To an observer, it seems that things are no longer changing. In reality, however, changes are continuously occurring at an atomic scale, but the changes balance each other out. When a system is at chemical equilibrium, the rate of the forward reaction is equal to the rate of the reverse reaction. Equilibrium is a property of the mixture, meaning that it does not matter how you start the process.
Exercises
Reading Questions
What does it mean when a system is in a state of dynamic equilibrium?
Why is there a limit as to how much salt you can dissolve?
Reason and Apply
Explain these observations in terms of the amounts of starting substances (AB) and products (A +B) in an equilibrium mixture.
Artificial sweetener is sweeter than the same amount of sucrose.
Much more sodium chloride, NaCl, dissolves in 1 L of water compared with silver chloride, AgCl.
Page 625When phosphorus pentachloride, PCl5(g), is placed in a sealed container, it breaks apart reversibly. The concentrations of the starting substance and products are monitored. Use the data in the table to create a graph showing the concentrations of the starting substance and products over time.
PCl5(g) ⇋ PCl3(g) + Cl2(g)
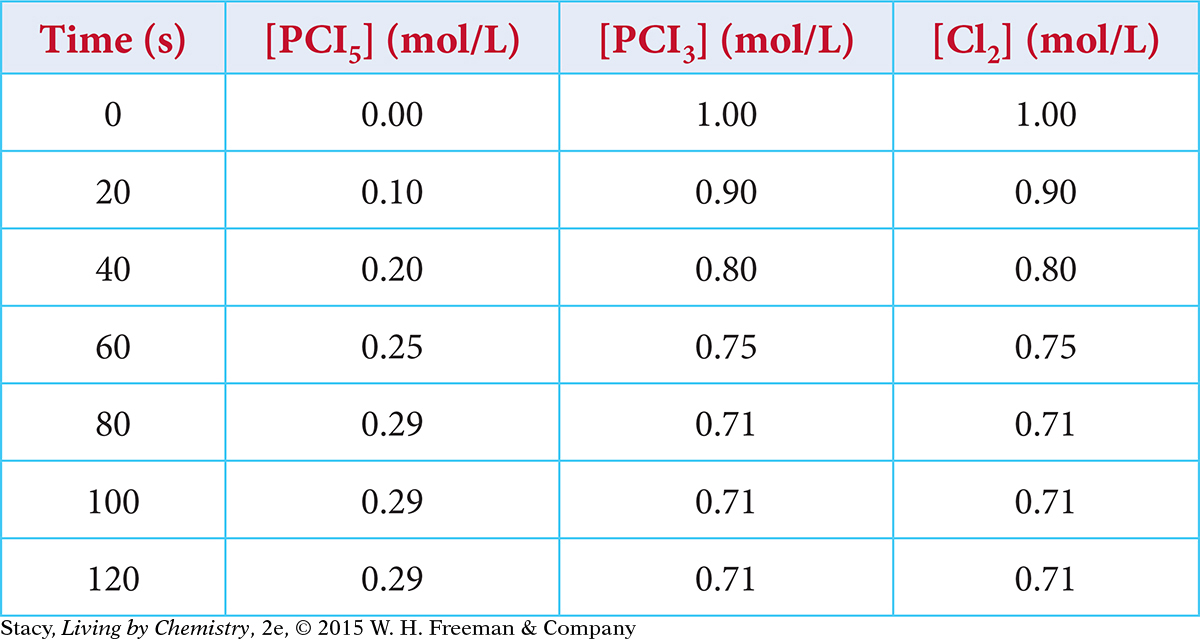
What are the equilibrium concentrations of the starting substance and the products?
At what time did the mixture reach equilibrium? How do you know?
At 20 seconds, which process is faster, the forward or the reverse process? At 100 seconds?
Why is the amount of starting substance, PCl5, not equal to the amounts of each of the products, PCl3 and Cl2, at equilibrium? Support your answer with evidence.
Why are the amounts of PCl3 and Cl2 always equal to one another?
The graph below shows the reversible separation of a molecule bound to a receptor.
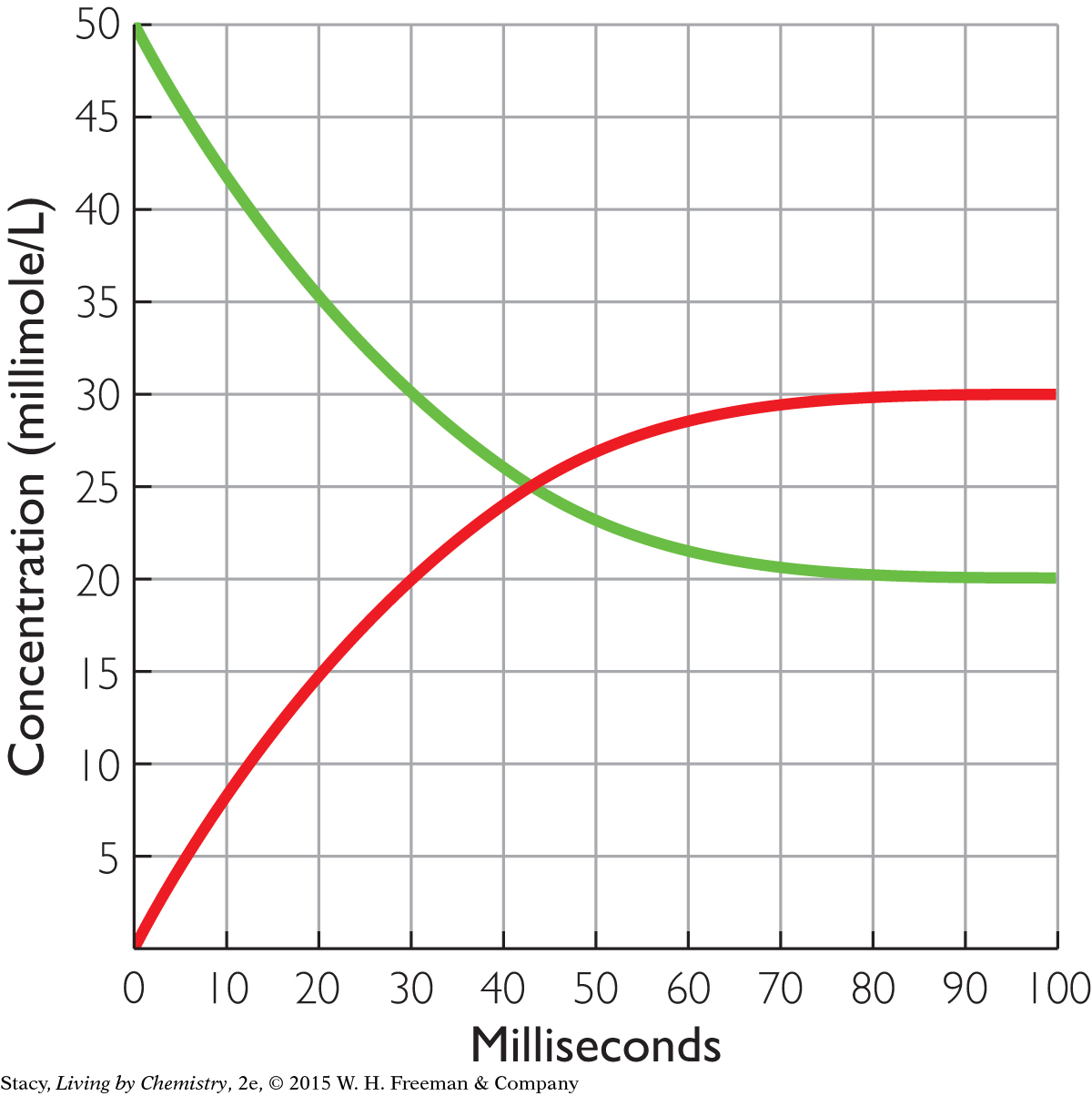
The process begins with all the molecules attached to receptors. Write a chemical equation to represent the process.
Label the two curves as either starting substance (with the molecule attached to the receptor) or products (molecule or empty receptor).
What are the equilibrium concentrations of the starting substance and the two products?
How long did it take for this process to reach equilibrium?
What is “equal” at equilibrium?