LESSON 6: A New Language: Chemical Names and Symbols
23
THINK ABOUT IT
CAREER CONNECTION
CAREER
CONNECTION
Jewelers can tell if a diamond is real by observing its properties, such as hardness, density, and how it bends light.

There are two bottles on a shelf in a chemistry lab. Each contains a substance that resembles diamonds. Bottle 1 is labeled C(s), and Bottle 2 is labeled ZrO2(s). Does either bottle contain diamonds? Do both bottles contain diamonds?
What do chemical names and symbols tell you about matter?
To answer this question, you will explore
The Language of Chemistry
Names and Symbols
Physical Form
The Language of Chemistry
EXPLORING THE TOPIC
The Language of Chemistry
Some chemical names are used in daily language, such as aluminum and iron, which cookware and machinery are made of, and ammonia, which is used for cleaning. Other chemical names, such as sodium chloride for salt and calcium carbonate for chalk, are used mainly by chemists.
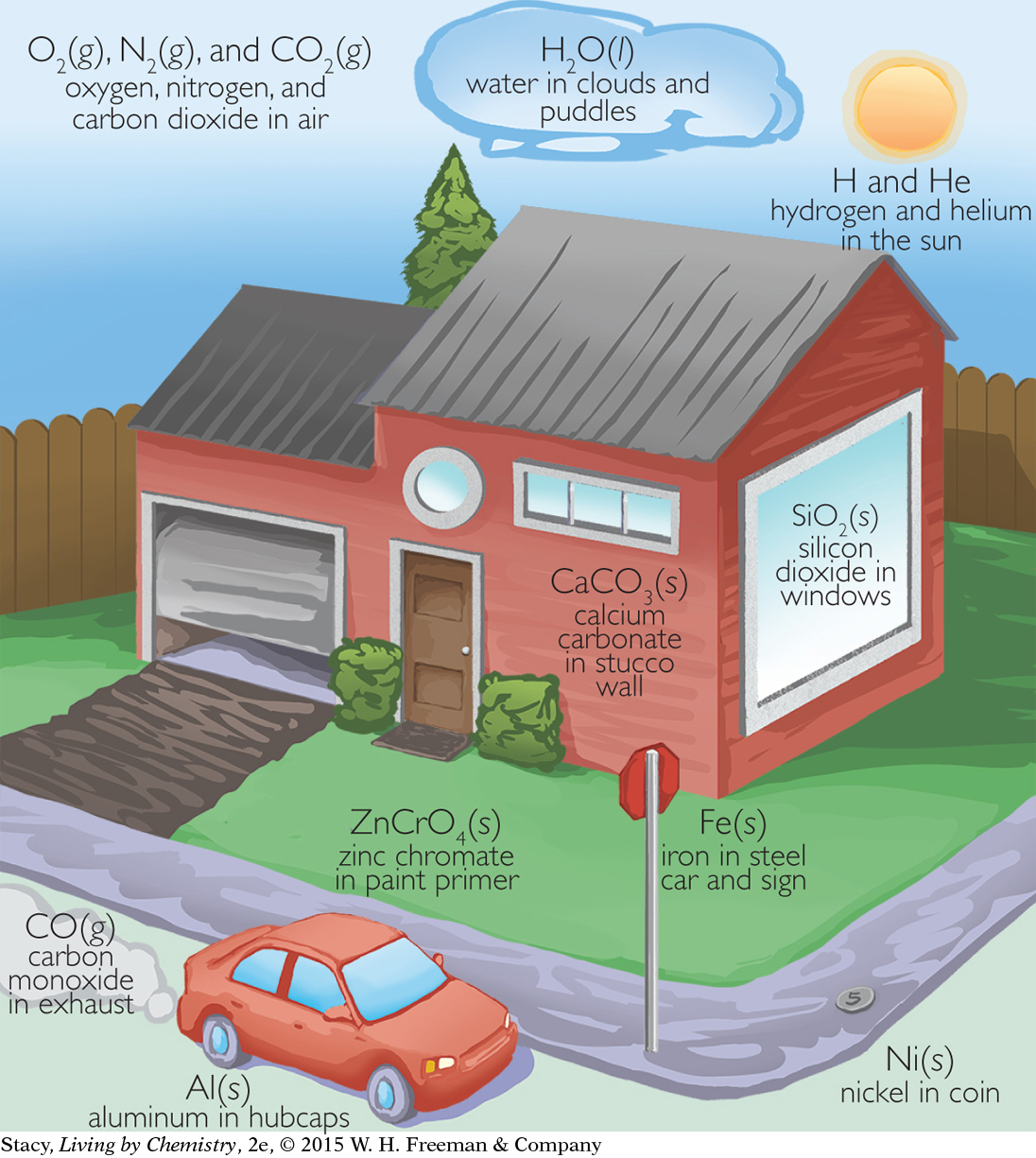
Names and Symbols
Names and Symbols
24
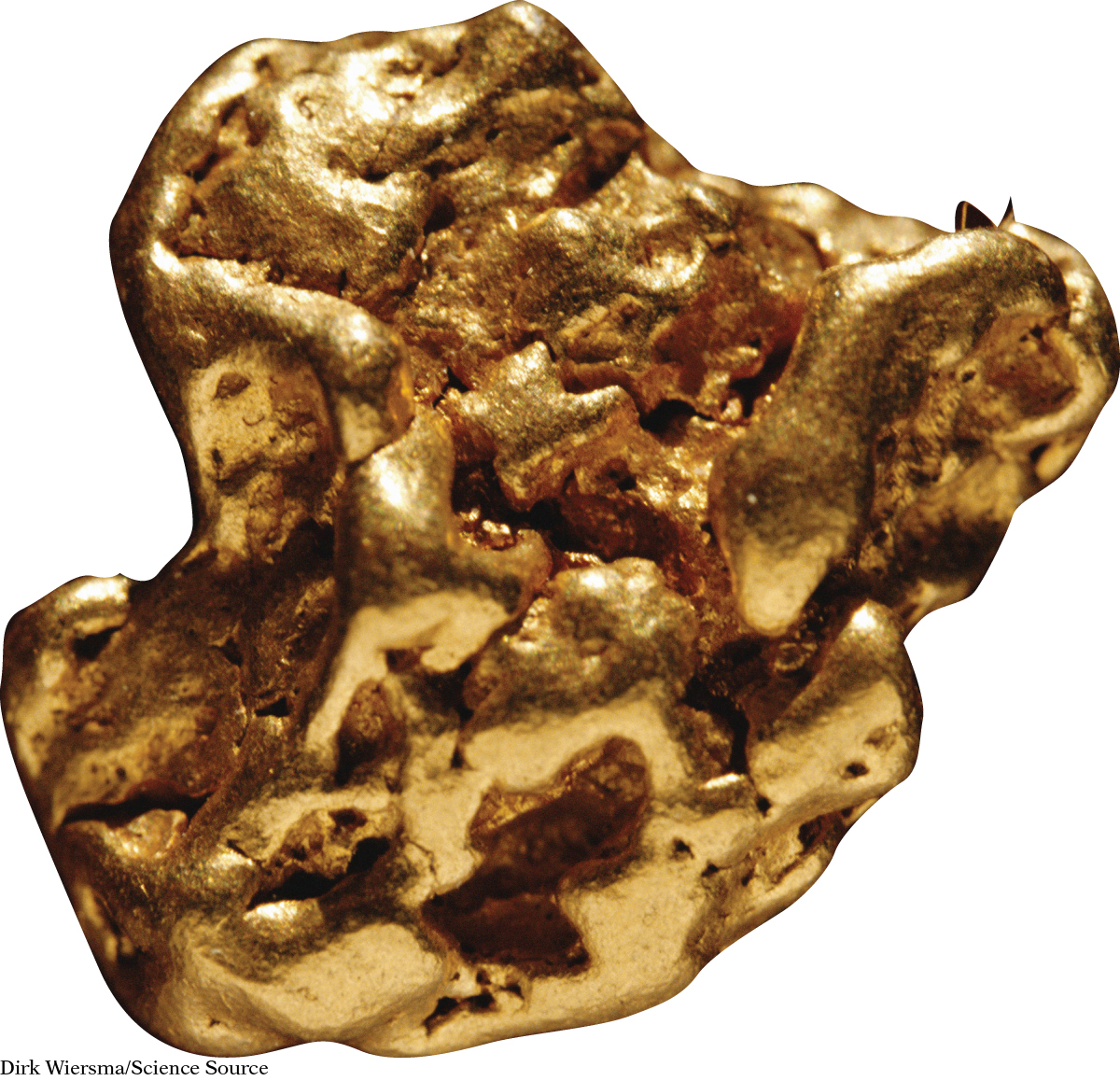
Elements are the building materials of all matter. In total, there are about 115 known elements. A few are shown here.
|
Metals |

Mercury, Hg
|

Copper, Cu
|
Gold, Au
|

Sodium, Na
|

Iron, Fe
|
|
Nonmetals |

Carbon, C
|

Chlorine, Cl
|

Iodine, I
|

Phosphorus, P
From top left: Harry Taylor/Getty Images, Dmitry Gool/iStockphoto/Thinkstock, Dirk Wiersma/Science Source, Martyn F. Chillmaid/Science Source, Sawayasu Tsuji/iStockphoto, Kae Deezign/Shutterstock, Charles D. Winters/Science Source, Andrew Lambert Photography/Science Source, Charles D. Winters/Science Source
|
Here are some things you might notice. The chemical symbols for the elements consist of one or two letters. The first letter is always capitalized. If there is a second letter, it is lowercase. An element can be a solid, liquid, or gas. Some elements are metals, some are not. Sometimes, the symbol for an element is an abbreviation for its name. Other times, the symbol comes from another source, such as a word in another language. For example, the symbol for iron is Fe from the Latin word ferrum, and the symbol for gold is Au from the Latin word aurum.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Neon is the gas used in neon signs. The gas is colorless but glows a bright orange-red when an electric current is run through it. Neon gas is put into glass tubes that have been bent and shaped to create colorful signs.

Elements combine in specific ratios to form compounds. A compound is represented by a chemical formula. For example, sodium chloride, or table salt, is NaCl, which tells you that salt is made of the elements sodium, Na, and chlorine, Cl, in a 1:1 ratio. The chemical formula for carbon dioxide is CO2. The subscript number “2” in the formula indicates that the elements carbon and oxygen are combined in a 1:2 ratio. (If the subscript is 1, you normally don’t write it.)

Compounds can differ greatly in appearance and behavior from the elements that they are composed of. For example, sodium is a shiny metal and chlorine is a gas. They are very different from sodium chloride, table salt.
Big Idea
Big Idea
All matter is made up of elements or compounds, or mixtures of these.
25
These minerals contain copper, Cu. Notice that not one of them is copper colored.

Copper oxide,Cu2O(s)
|

Copper sulfate,CuSO4(s)
|

Copper sulfide,CuS(s)
|
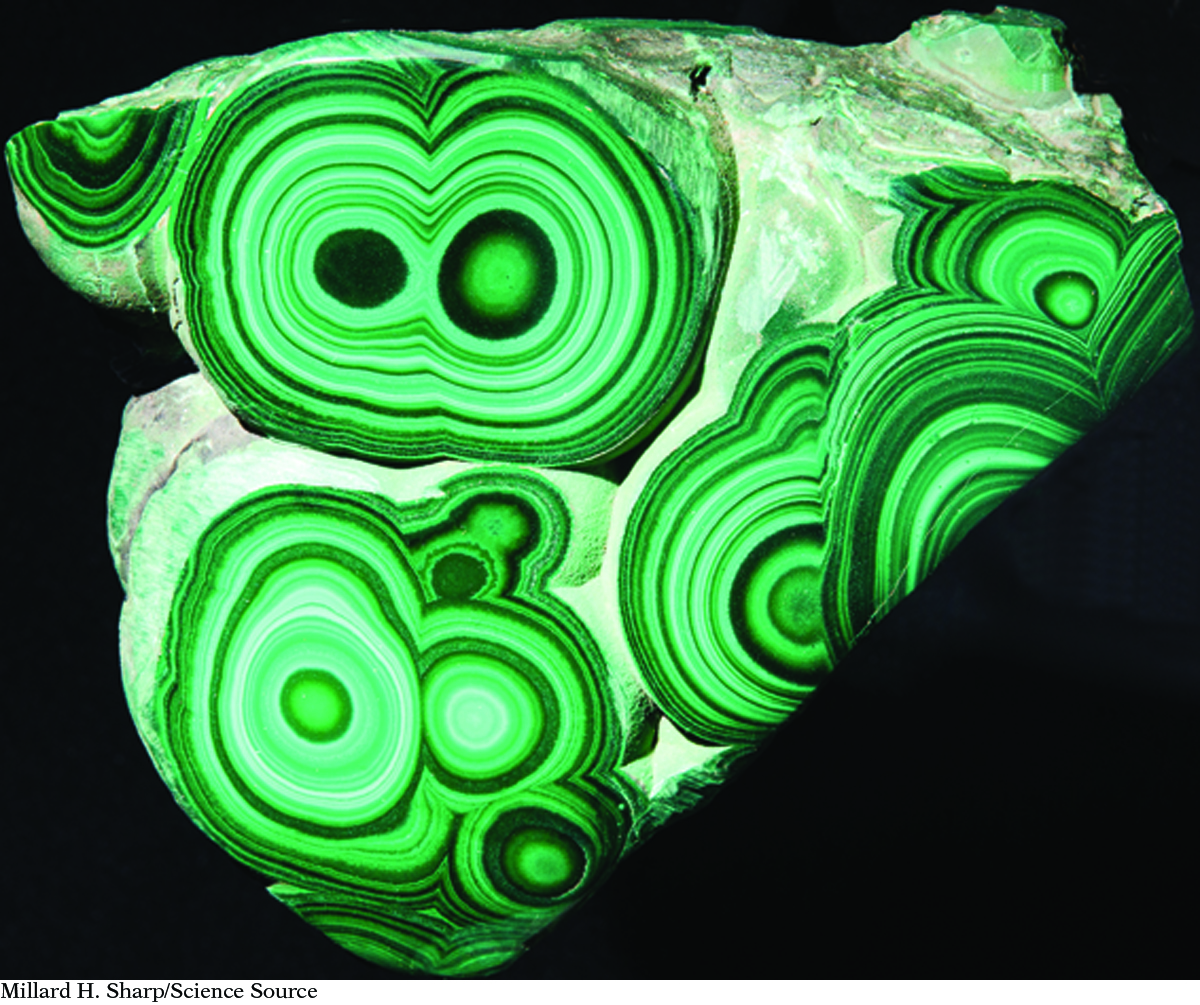
Copper carbonate hydroxide,Cu2(CO3)(OH)2(s)
From left: Andrew Lambert Photography/Science Photo Library, Ewa Brozek/iStockphoto, A. RIZZI/De Agostini Picture Library/Getty Images, Millard H. Sharp/Science Source
|
Physical Form
Physical Form

Elements and compounds can exist as solids, liquids, or gases. These forms are called the phases of matter, and are represented by using (s), (l), or (g) after the chemical formula. For example, water in the gas phase is written as H2O(g), water in the liquid phase is written as H2O(l), and water in the solid phase as ice is written as H2O(s).
There is another symbol for physical form, the symbol (aq) for aqueous. A substance is aqueous when it dissolves and forms a clear mixture with water. Many familiar liquids are actually aqueous solutions, such as grape juice, vinegar, and ocean water.
Important to Know
When solid sugar, C12H22O11(s), appears to “melt” in your mouth, it does not become liquid sugar, C12H22O11(l). It dissolves and becomes aqueous sugar, C12H22O11(aq).

Most substances are a mixture of compounds. For example, an orange is a mixture of water, H2O, fructose, C6H12O6, citric acid, C6H8O7, limonene, C10H16, and many other compounds. Notice that in mixtures, the components are not combined in a specific ratio. Some oranges are sweeter or more sour or more juicy because they have more fructose, citric acid, or water than other oranges. But the ratios of the elements in compounds never change. Water is always water, H2O.
LESSON SUMMARY
LESSON SUMMARY
What do chemical names and symbols tell you about matter?
KEY TERMS
element
chemical symbol
compound
chemical formula
phase
aqueous
All matter is composed of elements. Each element has a unique symbol. Elements combine with one another to form compounds. A chemical formula specifies which elements are present in a compound. The letters in chemical formulas are symbols for the elements, and the subscript numbers indicate the amount of each element in that substance. A chemical formula may also include the lowercase letters (s), (g), (l), and (aq), which stand for solid, gas, liquid, and aqueous.
26
Exercises
Reading Questions
Describe the difference between an element and a compound.
What is meant by physical form?
Reason and Apply
How many elements are included in the chemical formula for sodium nitrate, NaNO3? Name them.
What is the difference between NaOH(s) and NaOH(aq)?
You see a ring with a stone that looks like a diamond but wonder why it’s so cheap. The jeweler says the stone is a type of diamond called cubic zirconia. How can chemical symbols show that cubic zirconia is not a diamond?
You find two containers on a chemical shelf, one labeled Cu2O(s) and a second labeled CuO(s). Are these substances the same or different? Explain.