LESSON 10: Breaking the Code: The Periodic Table
THINK ABOUT IT
The elements copper, Cu, and gold, Au, share many similarities. Both are relatively unreactive elements. They are soft, so it is easy to bend and shape them. They are called coinage metals because they have been made into coins by many cultures. Copper and gold have high values as jewelry because they remain shiny for many years. Is the similarity in their properties related to their locations on the periodic table?
What information does the periodic table reveal about the elements?
To answer this question, you will explore
The Modern Periodic Table
Trends in Properties
The Modern Periodic Table
EXPLORING THE TOPIC
The Modern Periodic Table
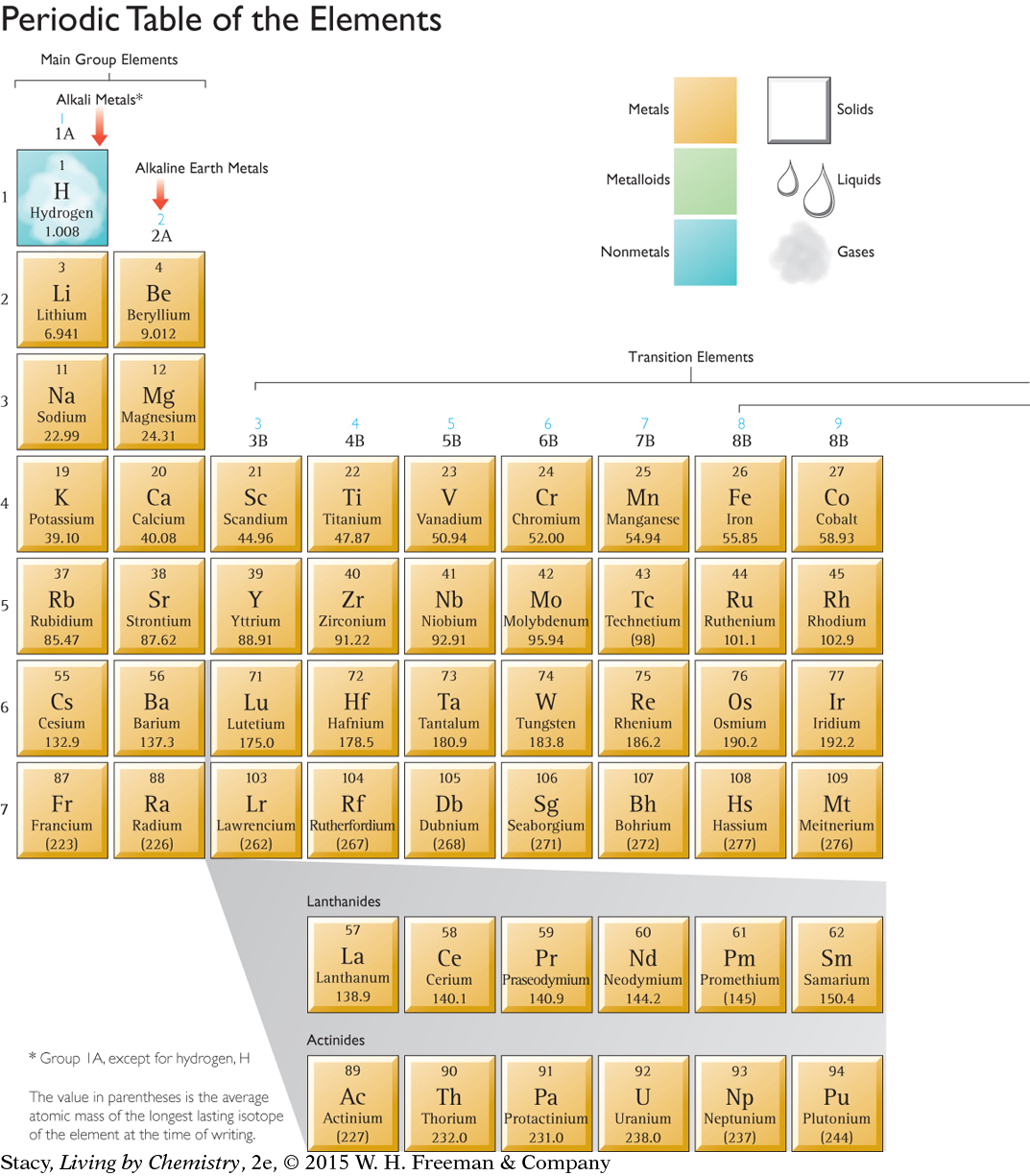
Scientists have detected around 115 different elements on the planet. Each is unique. Yet groups of elements have similar properties. Recall from Lesson 9: Create a Table that Dmitri Mendeleyev constructed a table based on patterns in the properties of the elements. His table has been replaced over the decades with many updated versions, such as the one shown on pages 42 and 43. The modern periodic table is a storehouse of valuable information about the elements. Over time, you will learn how to make use of the information that is contained there.
ELEMENT SQUARES
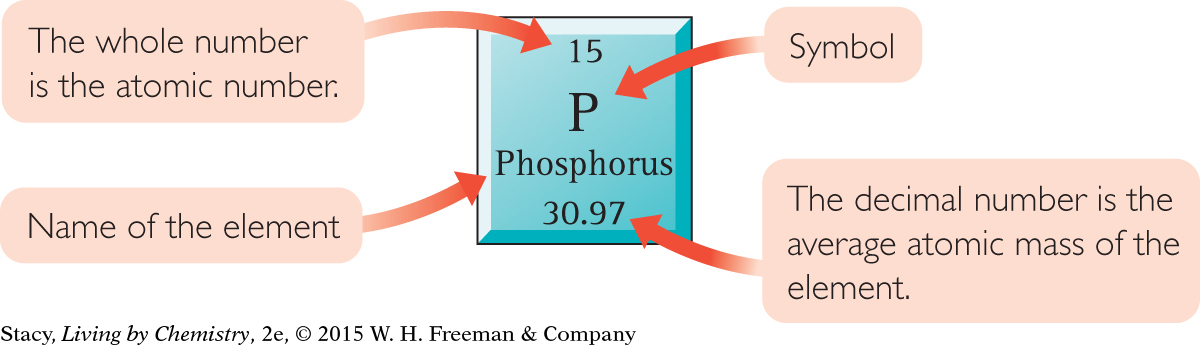
Each element has a square on the periodic table. Within each square is information about that element including its name and symbol. The whole number in each square is called the atomic number. Hydrogen is the first element in the table and has the atomic number 1. Helium is the second element and has the atomic number 2. Each atomic number corresponds to a different element.
The decimal number in each square on the periodic table is the average atomic mass in amu.
Here is a square from the periodic table.
PARTS OF THE PERIODIC TABLE
HEALTH CONNECTION
HEALTH
CONNECTION
Transition elements are important to human health. For example, iron is a central component of the protein hemoglobin, which helps to transport oxygen in the blood. Chromium helps our bodies metabolize glucose, and zinc helps to protect our immune systems.

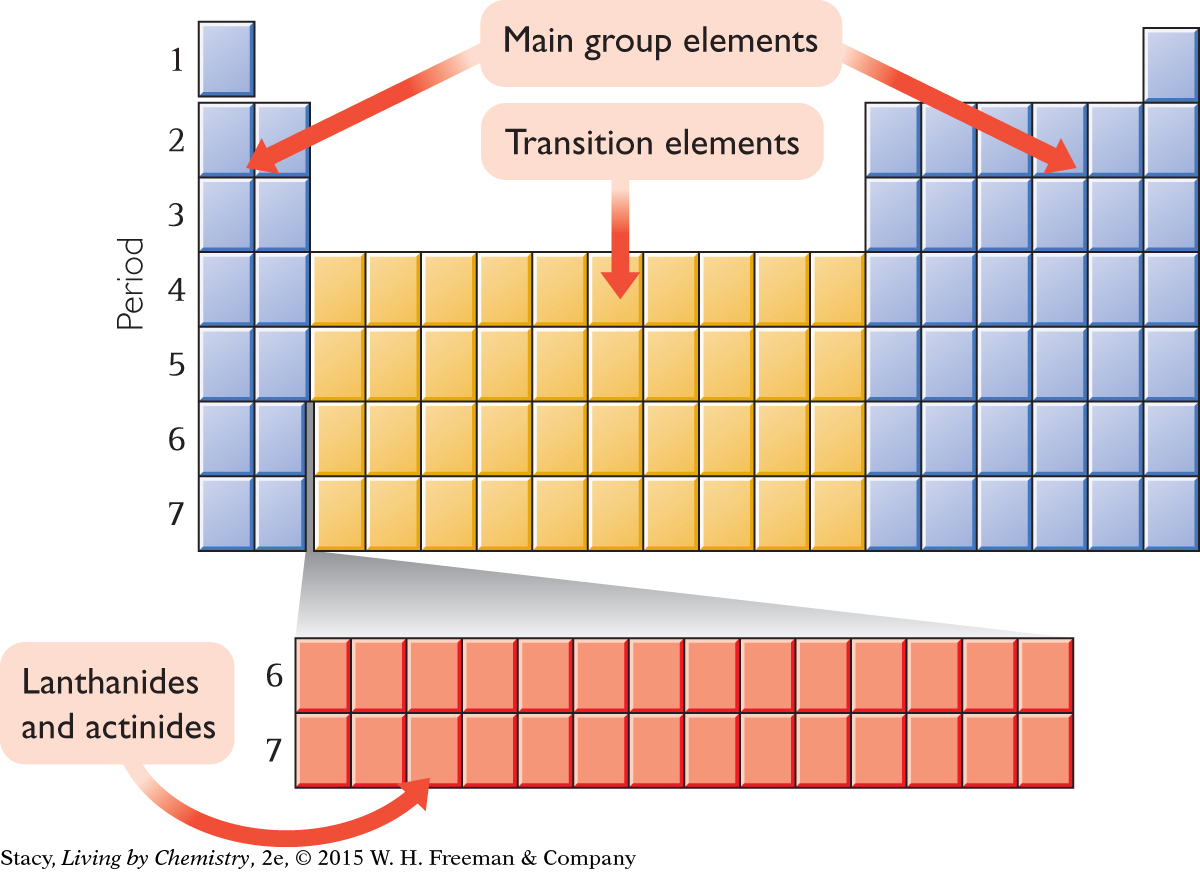
Most modern periodic tables have 18 vertical columns and 7 horizontal rows. The vertical columns are called groups, or families. Hydrogen, H, is in Group 1A, along with lithium, Li, and five other elements in that column. Some of the groups have specific names as shown below.
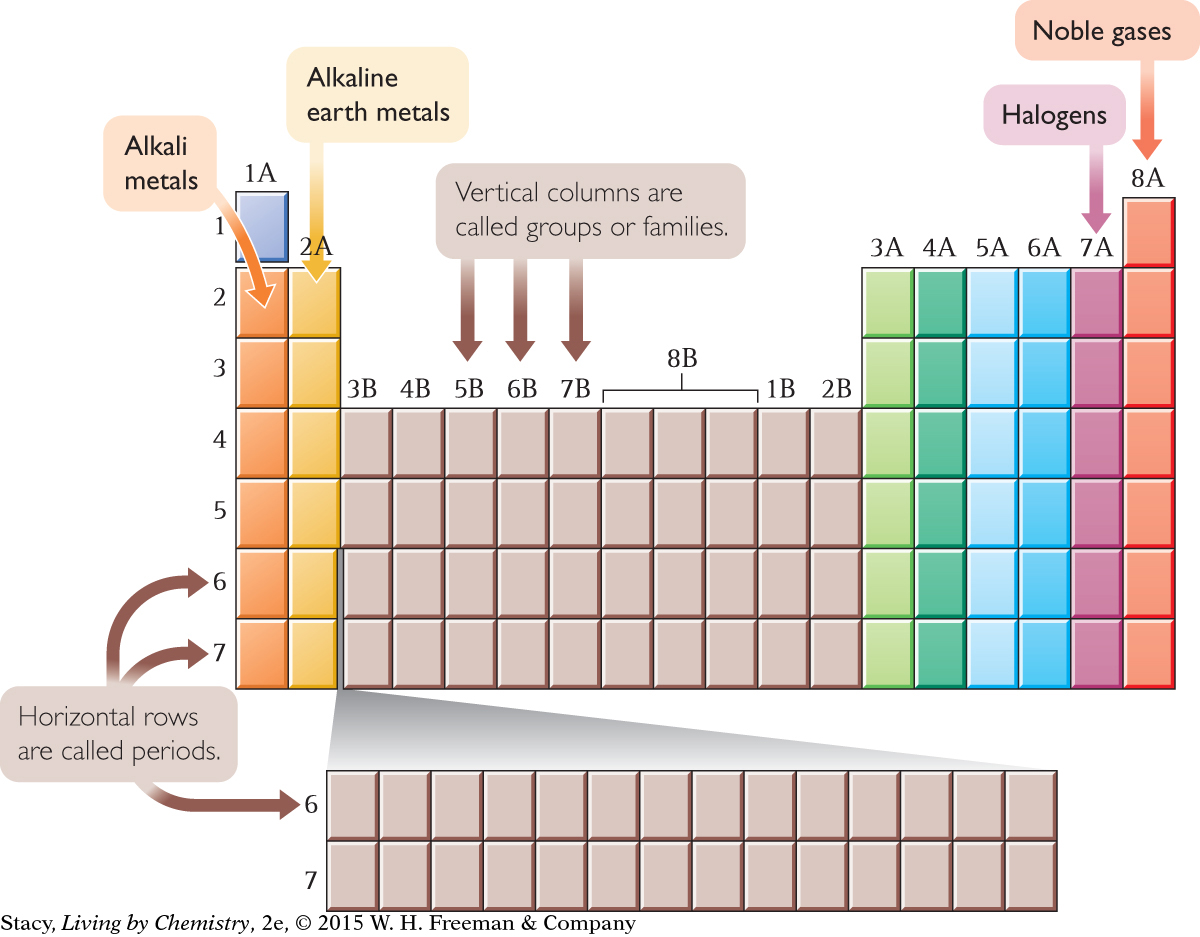
The horizontal rows of the table are called periods because patterns repeat periodically, or over and over again, in each row. There are only two elements in Period 1, hydrogen and helium. However, there are eight elements in Periods 2 and 3, and 18 elements in Period 4.
Chemists also have names for sections of the periodic table. Between Group 2A and Group 3A, for example, is where the transition elements fit in.
CAREER CONNECTION
CAREER
CONNECTION
Silicon Valley is a region in northern California that is known for its many high-technology and computer-based businesses. Silicon is a Group 4A element and a metalloid. It is the main component of semiconductor devices, such as the microchips used in computers and electronic equipment.

In addition, two rows of elements are usually shown at the bottom of the table. These elements are called the lanthanides and actinides. If you examine the atomic numbers of these elements, you’ll see that they belong in the sixth and seventh rows. If they were included where they belong, the table would look like this.
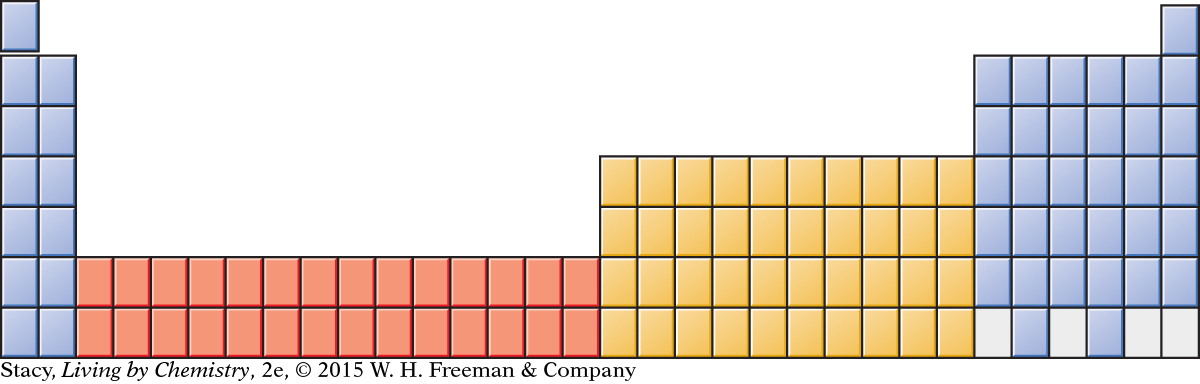
Most periodic tables show these two rows at the bottom so that everything will fit onto one page.
Trends in Properties
Trends in Properties
Once the elements are arranged according to their general properties, many other patterns or trends can be found. These three drawings illustrate some of the trends contained within the periodic table.
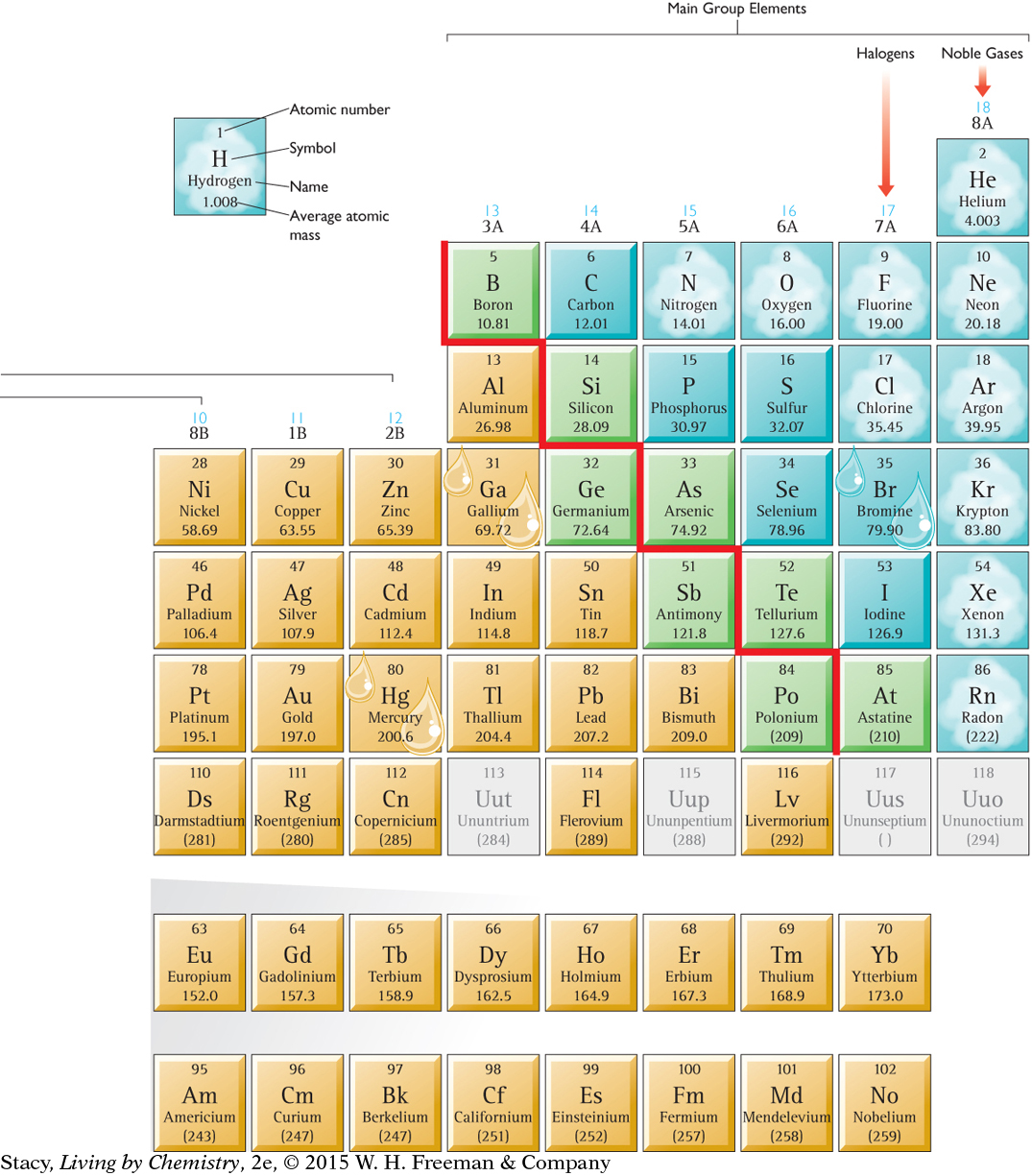
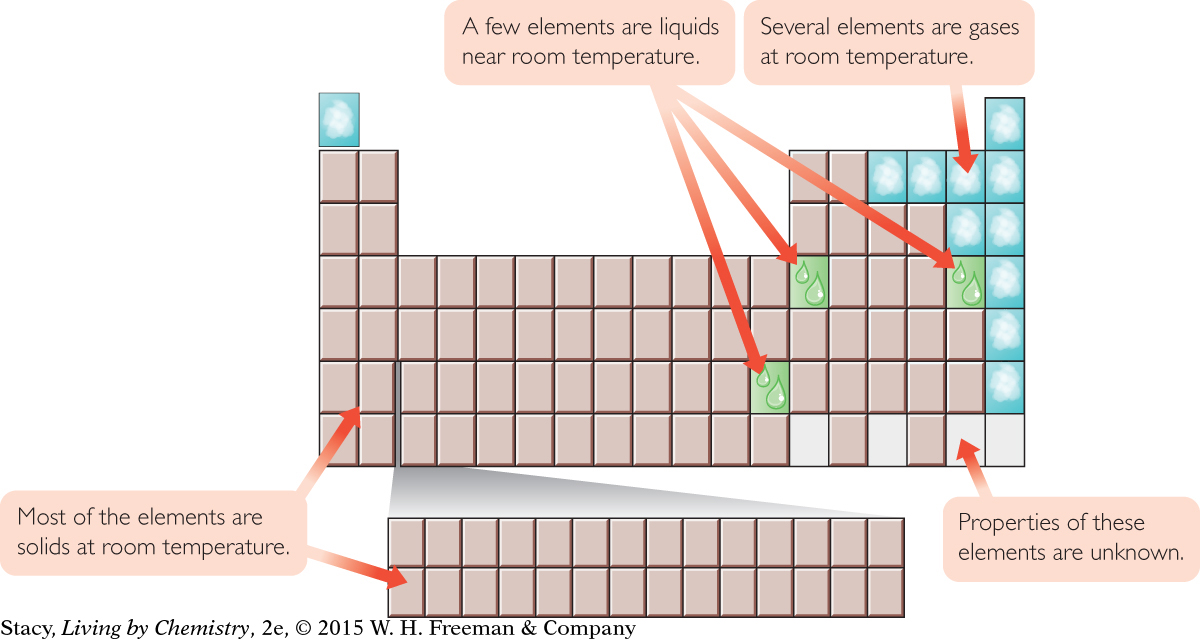
SOLIDS, LIQUIDS, AND GASES
Most of the elements are solids at room temperature. Several elements are gases at room temperature, but only a few are liquids at or near room temperature.
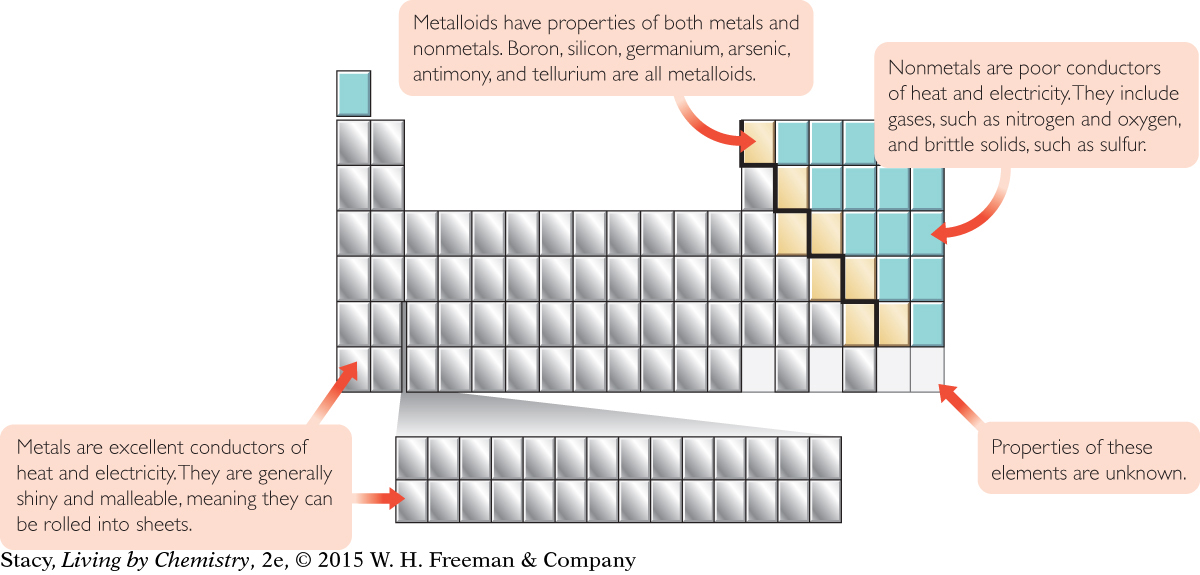
METALS, METALLOIDS, AND NONMETALS
The majority of the elements are metals. On most periodic tables, there is a stair-step line that divides the table. Metals are found to the left and nonmetals are found to the right of the stair-step line. The elements found along the stair-step line are called metalloids. Metalloids have properties like those of both metals and nonmetals.
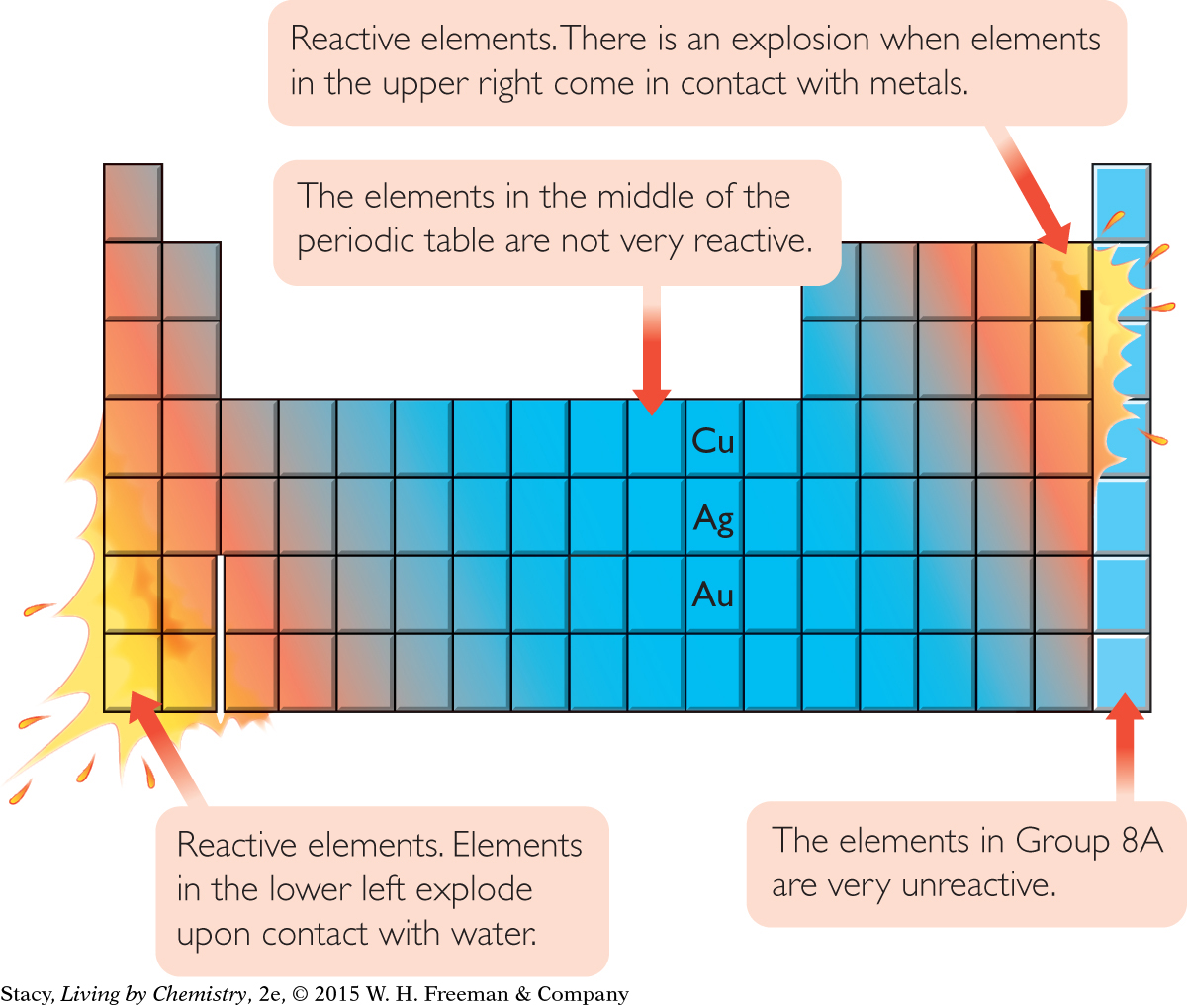
REACTIVITY
Elements in the lower left and upper right of the periodic table are the most reactive, with the exception of the noble gases in Group 8A, which are very unreactive. Copper, Cu, silver, Ag, and gold, Au, are metals that are in the middle of the periodic table and are not very reactive.
Example 1
Iodine, I
Find iodine, I, on the periodic table.
Find iodine’s atomic number, average atomic mass, period, and group.
Would you expect iodine to be a solid, liquid, or gas at room temperature?
Is iodine a metal, metalloid, or nonmetal? How can you tell?
Do you expect iodine to be reactive? Explain.
Solution
Iodine is in the lower-right area of the main group elements.
The atomic number is 53. Average atomic mass is 126.9 amu. Iodine is in Period 5 and Group 7A, halogens.
Iodine is a solid at room temperature.
Iodine is a nonmetal, because it is to the right of the stair-step line.
Yes, you can expect it to be reactive, though not as reactive as elements above it in Group 7A.
Example 2
Coinage Metals
Which element would make the best coin: phosphorus, P, silver, Ag, potassium, K, or xenon, Xe? Explain your thinking.
Solution
Xenon, Xe, is a gas, so it is definitely not a candidate for making a coin. Phosphorus, P, is a nonmetal that is dull and brittle. It would be difficult to shape into a coin. Silver, Ag, is a shiny, malleable metal, so it would make the best coin. Potassium, K, is also a metal, but it is too soft and reactive, so it would not make a good coin. A good coin should not react with other substances.
LESSON SUMMARY
LESSON SUMMARY
KEY TERMS
atomic number
group
alkali metal
alkaline earth metal
halogen
noble gas
period
main group elements
transition elements
lanthanides
actinides
metal
nonmetal
metalloid
What information does the periodic table reveal about the elements?
The periodic table is an organized chart of the elements. Each element square contains valuable information, including the element name, symbol, atomic number, and average atomic mass. These elements are arranged in vertical columns called groups, or families, and horizontal rows called periods. Most elements are solids and metals, except for those in the upper right of the table. The most reactive elements are located in the lower left and upper right of the table, excluding the noble gases, which are unreactive and located in the last column on the right.
Exercises
Reading Questions
Describe how reactivity changes as you go down Group 1A.
Choose two different properties and describe how they vary across a period.
Reason and Apply
You will need a handout of the periodic table.
On your periodic table, clearly label the alkali metals, the alkaline earth metals, the halogens, and the noble gases. (If you wish, you may color them and provide a color key at the top.)
Label the main group elements, the transition elements, and the lanthanides and actinides.
Name two elements that have properties similar to those of beryllium, Be, and have average atomic masses higher than 130.
Which of these elements are solids at room temperature?
fluorine, F
titanium, Ti
lead, Pb
oxygen, O
potassium, K
silicon, Si
Which of these elements are nonmetals?
bromine, Br
carbon, C
boron, B
thallium, Tl
phosphorus, P
aluminum, Al
Which two of these elements are the least reactive? Explain your thinking.
chlorine, Cl
barium, Ba
copper, Cu
rubidium, Rb
potassium, K
mercury, Hg
Can you make jewelry out of each of the elements listed below? Explain your thinking.
copper, Cu
neon, Ne
sodium, Na
platinum, Pt