LESSON 14: Isotopia: Stable and Radioactive Isotopes
THINK ABOUT IT
Some isotopes are found in nature and others exist only because they were created in the laboratory or under unusual conditions. If you went digging in a copper mine and analyzed the samples, you would find copper atoms with either 34 or 36 neutrons. But do any other isotopes of copper exist?
What types of isotopes do the various elements have?
To answer this question, you will explore
Naturally Occurring Isotopes
Stable and Radioactive Isotopes
Naturally Occurring Isotopes
EXPLORING THE TOPIC
Naturally Occurring Isotopes
When an atom or element can be found somewhere on Earth, it is called naturally occurring. Most chemists agree that there are about 92 naturally occurring elements on the periodic table. The rest of the elements on the table have existed only as a result of human activity or unusual circumstances.
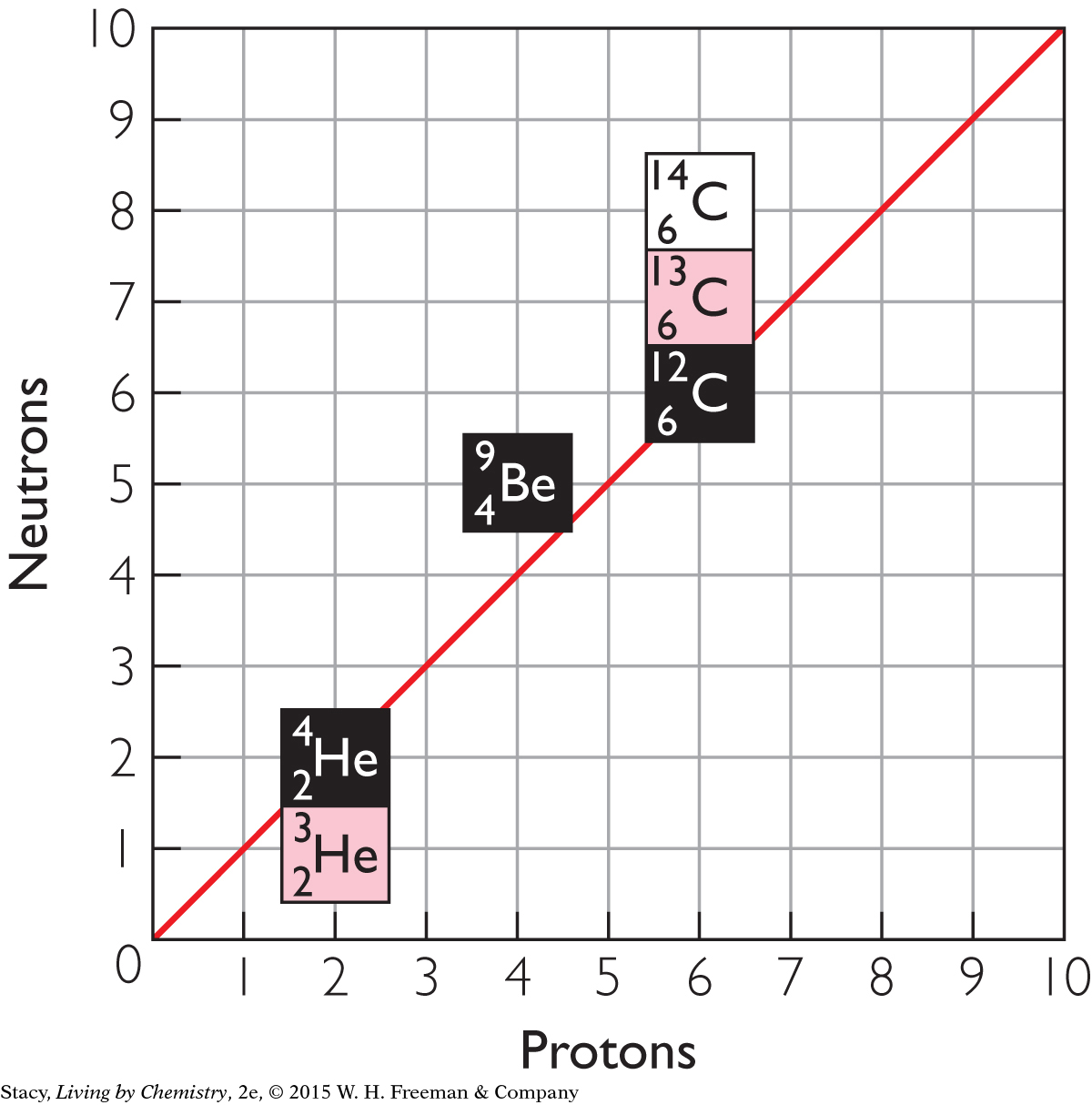
The element beryllium, Be, has only one naturally occurring isotope. This means all beryllium atoms have four protons and five neutrons. The element helium, He, has two naturally occurring isotopes. Its atoms have two protons and either one or two neutrons. Helium and beryllium are shown plotted on a graph of neutrons versus protons. Notice that helium-4 lies on the diagonal line, because its neutrons and protons are equal in number.
Now consider an element like carbon, C, with more than one isotope. Its isotopes, carbon-12, carbon-13, and carbon-14, are also on the graph. Notice that carbon’s isotopes line up vertically. This is because they all have 6 protons. One of carbon’s isotopes, with 6 protons and 6 neutrons, lies on the diagonal. The other two isotopes have more neutrons than protons in their nuclei.
ISOTOPES OF THE FIRST 95 ELEMENTS
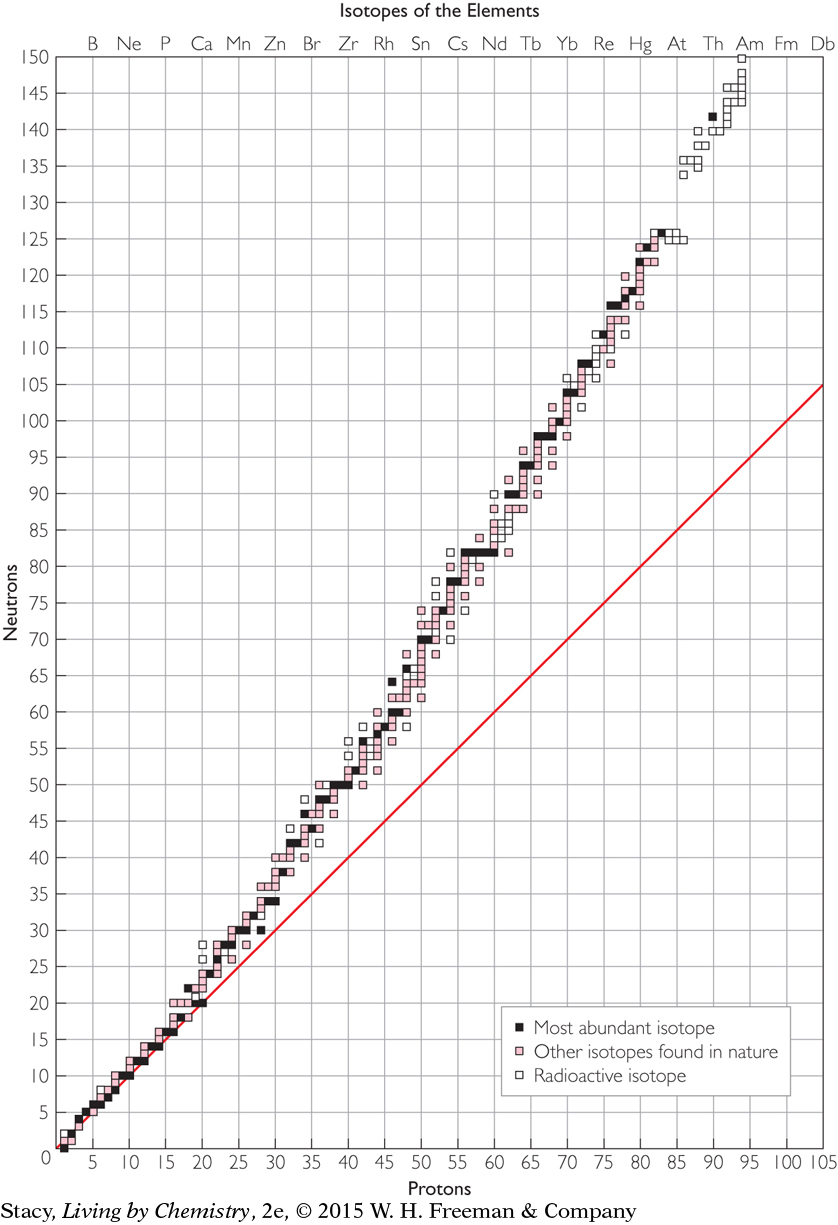
The graph below has been expanded to include all of the isotopes of the first 95 elements. As before, each square plotted on the graph represents a different isotope. Take a few minutes to study the graph.
TECHNOLOGY CONNECTION
TECHNOLOGY
CONNECTION
Technetium, Tc, and promethium, Pm, are not found in nature. They are human-made isotopes that are very unstable. Two forms of technetium, though, can be used as radioactive tracers in living tissues for research and for diagnosis.
Here are some things you might notice:
TECHNOLOGY CONNECTION
TECHNOLOGY
CONNECTION
Rubidium-strontium dating is a method used to determine the age of geological and lunar rock samples. It is based on the fact that rubidium-87 decays over time to become strontium-87.

Some elements have only one isotope. However, most elements have more than one isotope.
The squares that lie on the diagonal line represent isotopes with equal numbers of protons and neutrons.
Except in hydrogen-1 and helium-3 atoms, the number of neutrons in an atom’s nucleus is always equal to or greater than the number of protons. So most of the points lie on or above the diagonal line.
Page 67Radioactive isotopes are not common. (You will learn more about radioactivity later in this lesson.)
No element has more than 10 isotopes. Most elements have between 1 and 6 isotopes.
The number of neutrons is roughly equal to the number of protons for atoms up to atomic number 20.
The collection of plotted points curves up, away from the diagonal line. Beyond atomic number 20, elements have considerably more neutrons than protons.
The majority of radioactive isotopes are elements with atomic numbers above 80.
The elements with even numbers of protons have more isotopes than elements with odd numbers of protons. Even numbers of neutrons are also more common than odd numbers.
Stable and Radioactive Isotopes
Stable and Radioactive Isotopes
When atoms are not stable, they emit small particles. This means that small bits of the nucleus come flying out of the atom. These less stable isotopes are called radioactive isotopes. They decay over time as particles are spontaneously emitted from the nucleus in a process called radioactive decay. You will learn more about radioactive decay in upcoming lessons.
As you saw in the graph, hydrogen, H, argon, Ar, and cerium, Ce, are examples of elements that have radioactive isotopes shown with white squares. There are only a few white squares scattered throughout the graph until you reach polonium, Po, element number 84. However, technetium, Tc, promethium, Pm, and every element from polonium and beyond, have only radioactive isotopes.
STABILITY STARTS IN THE NUCLEUS
A stable isotope has a stable nucleus. Stability is related to the balance between the number of protons and the number of neutrons in the nucleus. An atom that doesn’t have enough neutrons will disintegrate. So will an atom with too many neutrons. The larger the atom, the more neutrons it takes to make a stable nucleus. For example, isotopes of element number 74, tungsten, W, require at least 110 neutrons to be stable.
Example
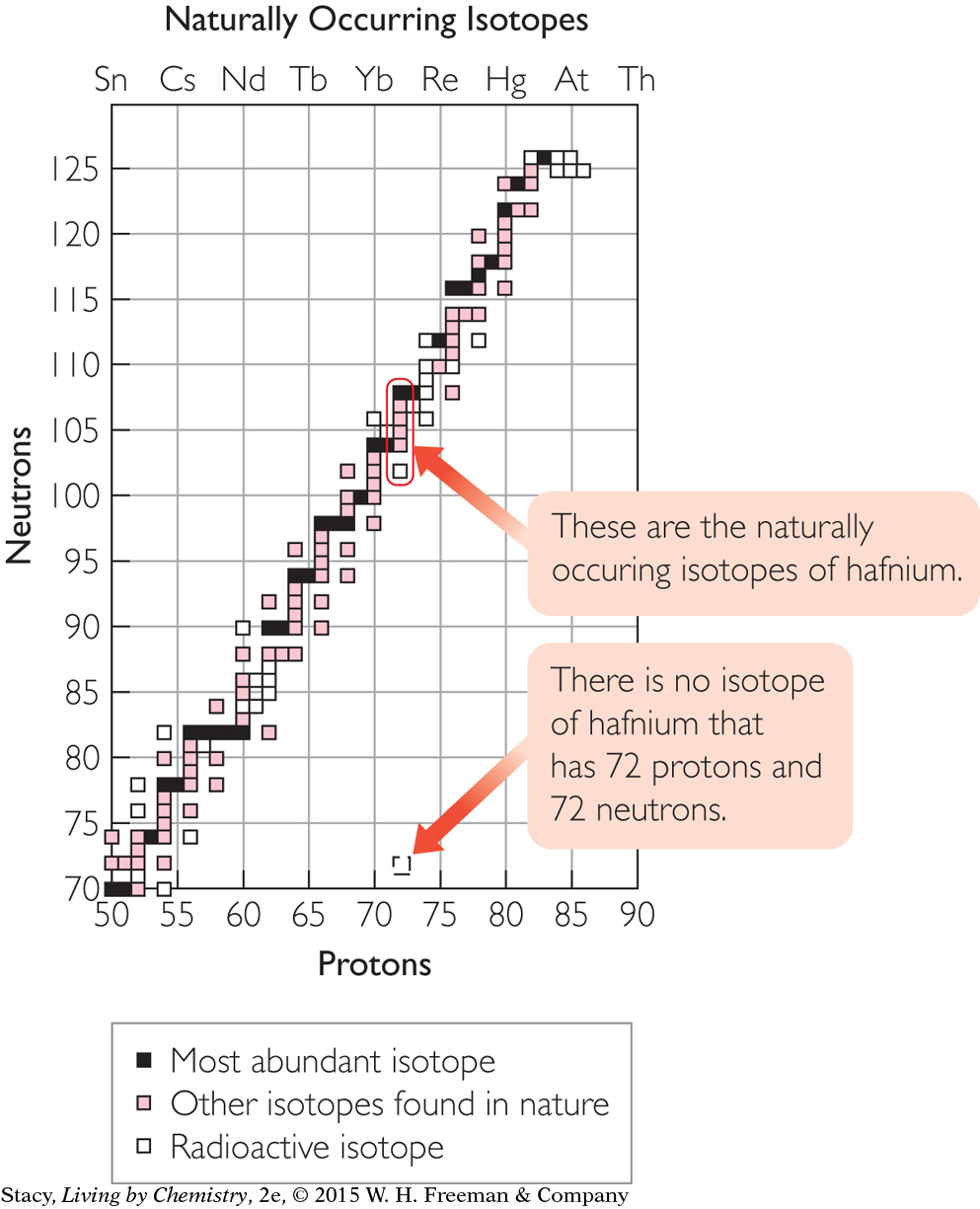
Hafnium-144
Do you expect the isotope hafnium-144 to exist in nature? Explain your thinking.
Solution
The task here is to find out if there is a square on the isotope graph that corresponds to hafnium-144. The way to do this is to figure out how many protons and neutrons are in hafnium-144. Use the periodic table to find the atomic number of hafnium, Hf. The atomic number is 72, so a hafnium atom has 72 protons. An isotope of hafnium with a mass of 144 has 144 – 72 = 72 neutrons. There is no square on the graph corresponding to 72 protons and 72 neutrons. So a hafnium atom with only 72 neutrons does not exist in nature.
LESSON SUMMARY
LESSON SUMMARY
What types of isotopes do the various elements have?
KEY TERM
radioactive isotope
Most elements have more than one naturally occurring isotope. Most of these isotopes are stable. Stable isotopes have stable nuclei, with just the right balance of protons and neutrons. In atoms with atomic numbers up to 20, the number of neutrons is roughly equal to the number of protons. Atoms with atomic numbers between 20 and 84 require progressively more neutrons in the nucleus to attain stability. Unstable isotopes are called radioactive isotopes. The nucleus of an unstable isotope will decay and emit radioactive particles. All elements beyond atomic number 83 are unstable, and therefore, all their isotopes are radioactive.
Exercises
Reading Questions
Explain the relationship between the words atom and element.
Explain the relationship between the words atom and isotope.
Reason and Apply
Find these elements on the isotope graph on page 66. How many stable isotopes does each element have?
oxygen, O neodymium, Nd copper, Cu tin, Sn Page 69Use the isotope graph to determine which of these isotopes would be found in nature.
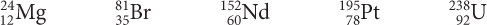
What does the diagonal line on the graph represent?
Use the isotope graph to find the isotopes described below. In each case, give the isotope name and the isotope symbol.
Find three isotopes with equal numbers of neutrons and protons.
Find two isotopes with the same number of protons.
Find two isotopes with the same number of neutrons.
Find two isotopes with the same atomic mass units.
Is an atom with a nucleus of 31 protons and 31 neutrons a stable isotope? Why or why not?
Draw basic atomic models for all the stable isotopes of oxygen, O.
Name five elements on the periodic table that have only radioactive isotopes. Determine the neutron-to-proton ratio for each of their isotopes, in decimal form.
How many protons could a stable atom with 90 neutrons have? Which elements would these be?
Would an atom with 60 protons and a mass number of 155 be stable?
Which of the following isotopes are likely to be found in nature? Identify the element by name if it is an isotope that might be found in nature.

Choose the best answer. The diagonal line on the isotope graph on page 66 represents nuclei with
the same number of protons.
the same number of neutrons.
the same number of protons as neutrons.
the same number of protons plus neutrons.
Choose the best answer. In general, elements with an even atomic number
have only one isotope.
have more isotopes than those with an odd atomic number.
have the same number of protons as neutrons.
are halogens.