LESSON 22: Isn’t It Ionic?: Polyatomic Ions
THINK ABOUT IT
So far, we have considered ionic compounds made of only two elements, a metal and a nonmetal. However, there are some ionic compounds that consist of more than two elements. For example, sodium hydroxide, NaOH, which is commonly found in drain cleaner, consists of one metal element and two nonmetal elements. You might wonder about the O and the H. Are they both ions?
What is a polyatomic ion?
To answer this question, you will explore
Polyatomic Ions
Predicting Chemical Formulas for Polyatomic Ions
Polyatomic Ions
EXPLORING THE TOPIC
Polyatomic Ions
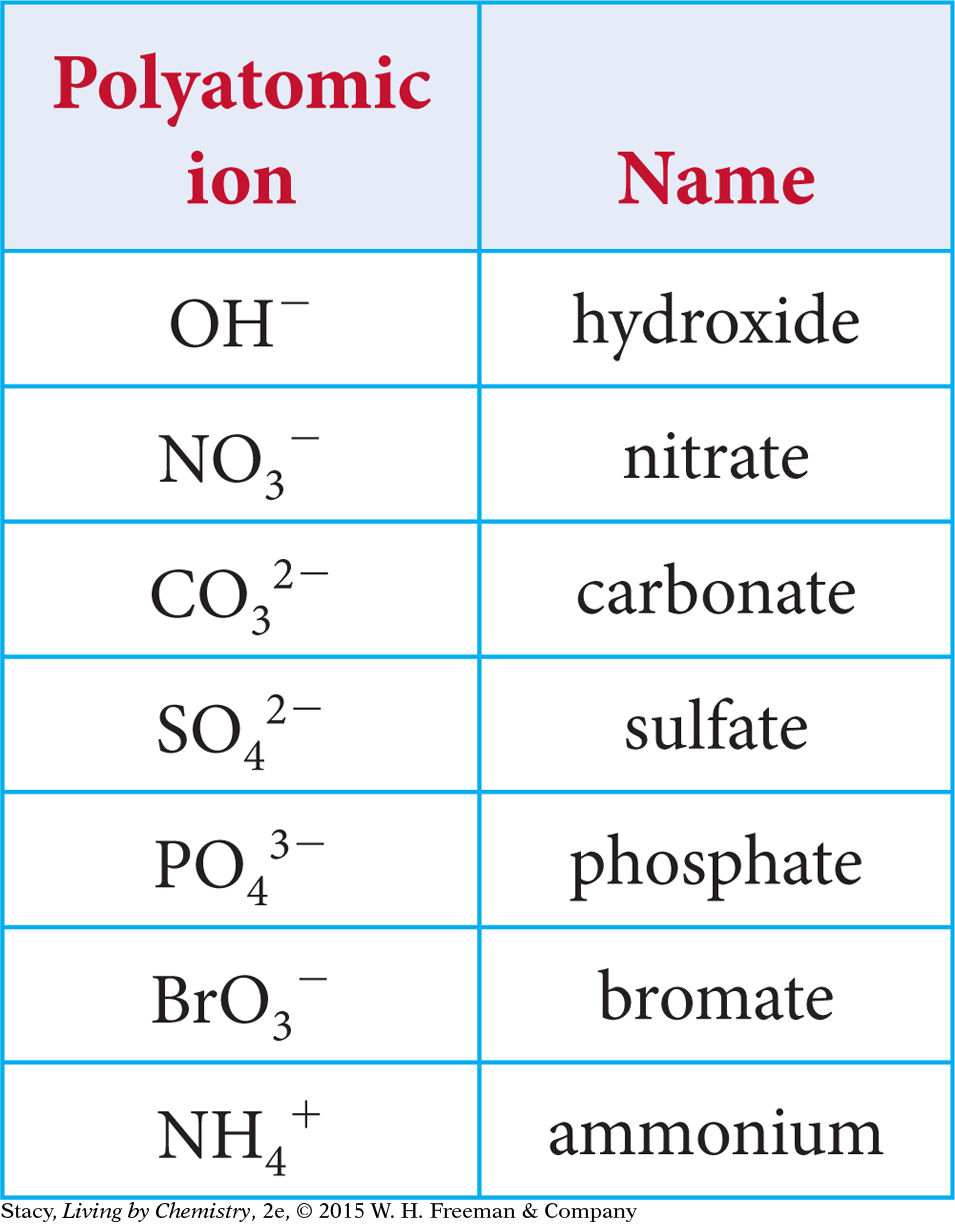
Some ionic compounds contain ions that consist of two or more elements. These ions are called polyatomic ions. In contrast, ions that have only one element are called monatomic ions. Mono means “one” and poly means “many.” Calcium sulfate, CaSO4, is an example of a compound made up of a monatomic ion and a polyatomic ion. The calcium ion is monatomic and the sulfate ion is polyatomic.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Sodium hydroxide, or lye, has many uses. It is used to straighten or curl hair, but if left in too long, it can damage hair and skin.

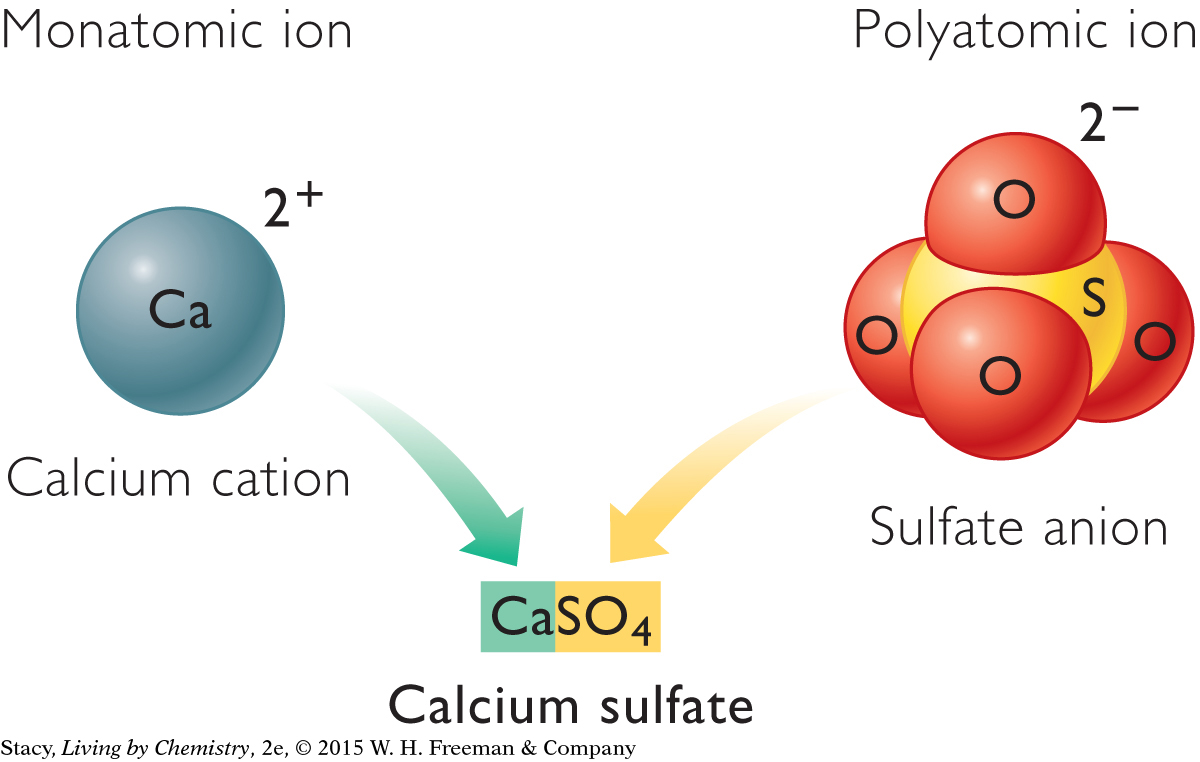
It is important to keep in mind that polyatomic ions are a group of atoms that stay together. In calcium sulfate, the entire group of atoms in the sulfate ion has a negative charge.
NAMES OF POLYATOMIC IONS
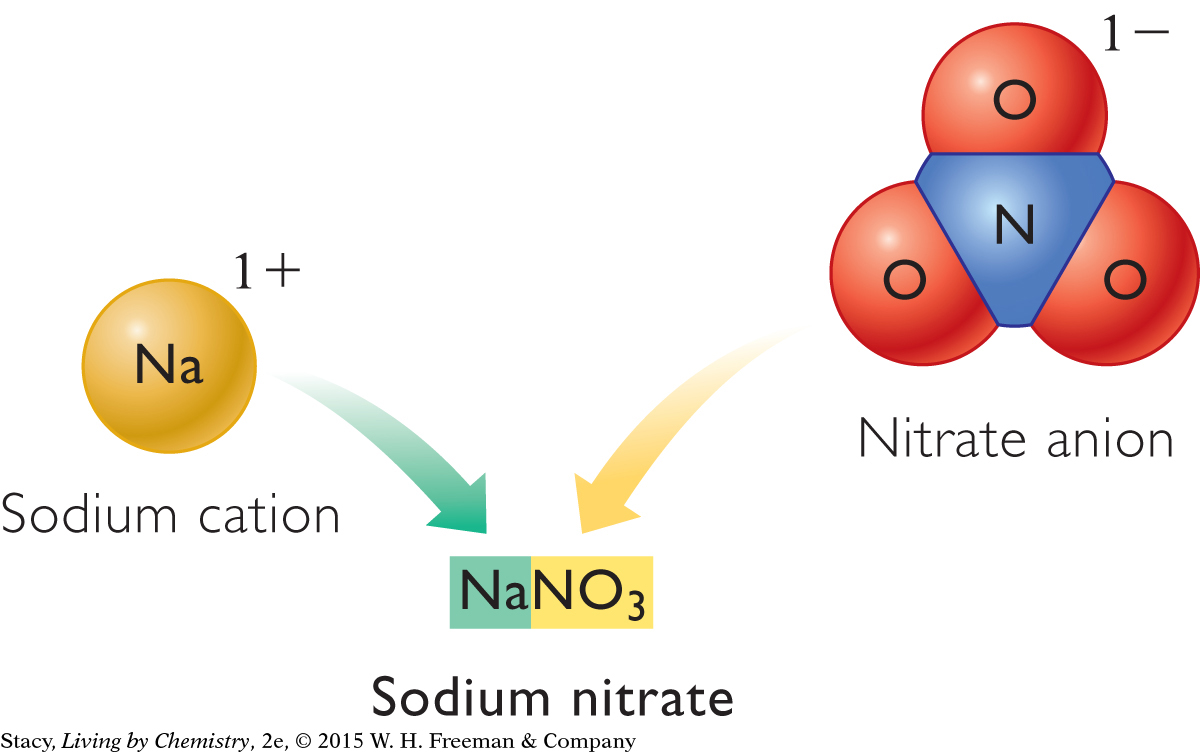
Ionic compounds with polyatomic ions follow specific naming rules. Each polyatomic ion has its own name, as shown in the table. When naming a compound, you simply insert the polyatomic ion name at either the beginning or ending of the chemical name. The cation is first and the anion is second. For example, a compound that ends with NO3 is a nitrate. NaNO3 is called sodium nitrate, and Ca(NO3)2 is called calcium nitrate.
Predicting Chemical Formulas for Polyatomic Ions
Predicting Chemical Formulas for Polyatomic Ions
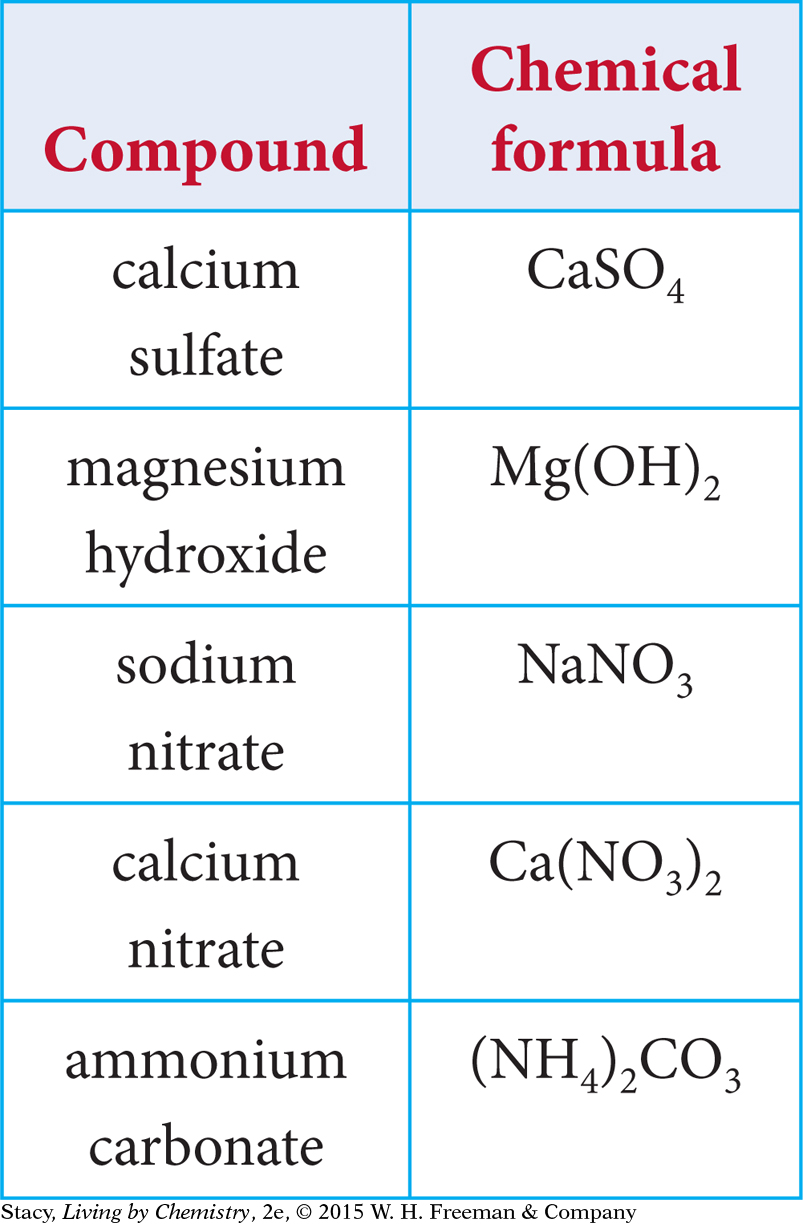
Several chemical formulas with polyatomic ions are listed in the table. Notice that in some cases there are parentheses around the polyatomic ion, with a subscript after the second parenthesis. Why are the formulas written this way? How do you determine the subscript?
Recall that you can use the rule of zero charge to determine the chemical formulas associated with ionic compounds. For example, a single calcium ion combines with a single sulfate ion because the charges add up to zero:
Ca2+ + SO42– becomes CaSO4, calcium sulfate
Important to Know
A polyatomic ion is treated as a unit. For example, the formula for calcium nitrate is written as Ca(NO3)2 with parentheses around NO3. Notice that it is not written as CaN2O6.
However, like simple ionic compounds, compounds with polyatomic ions do not always combine in a 1:1 ratio. When there is more than one of the same polyatomic ion in a formula, the ion is enclosed in parentheses and a subscript number indicates how many ions are in the compound. For example, a single calcium ion combines with two nitrate ions:
Ca2+ + NO3– + NO3– becomes Ca(NO3)2, calcium nitrate
Below are two more examples of ionic compounds containing polyatomic ions. Each of these compounds consists of three ions. One compound has monatomic and polyatomic ions, while the other has only polyatomic ions. The rule of zero charge applies to both.
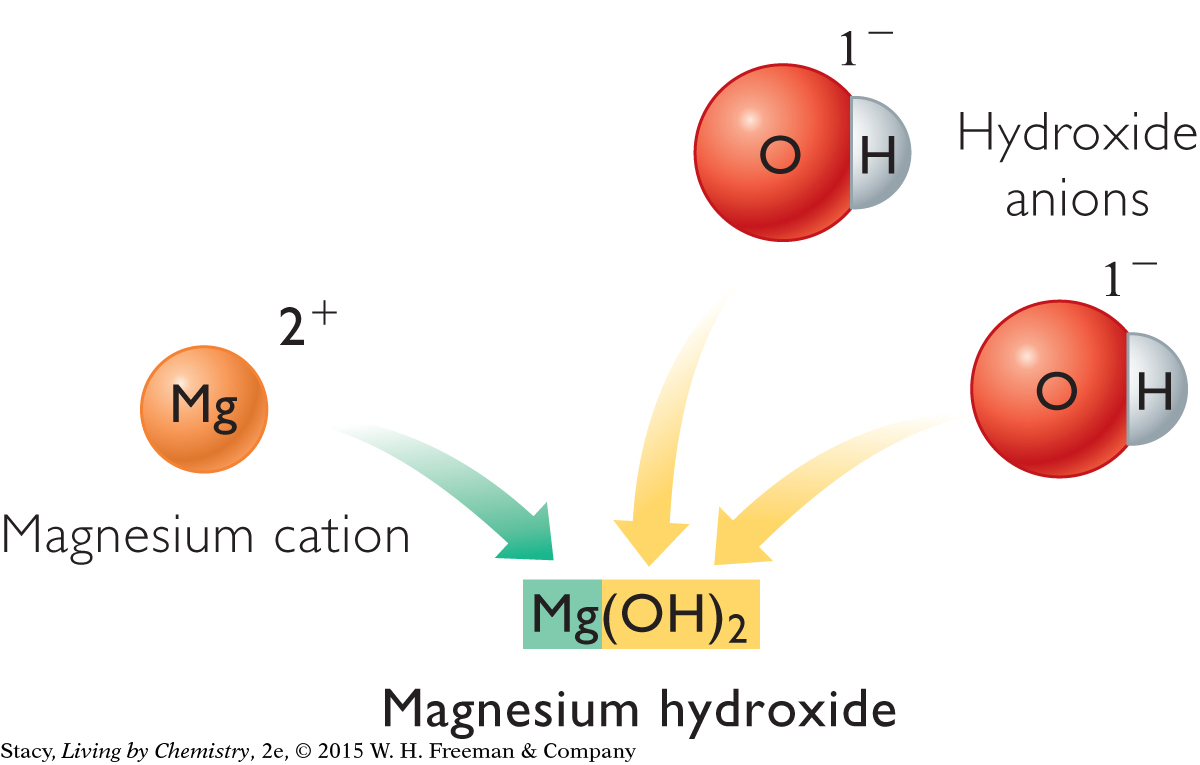
|
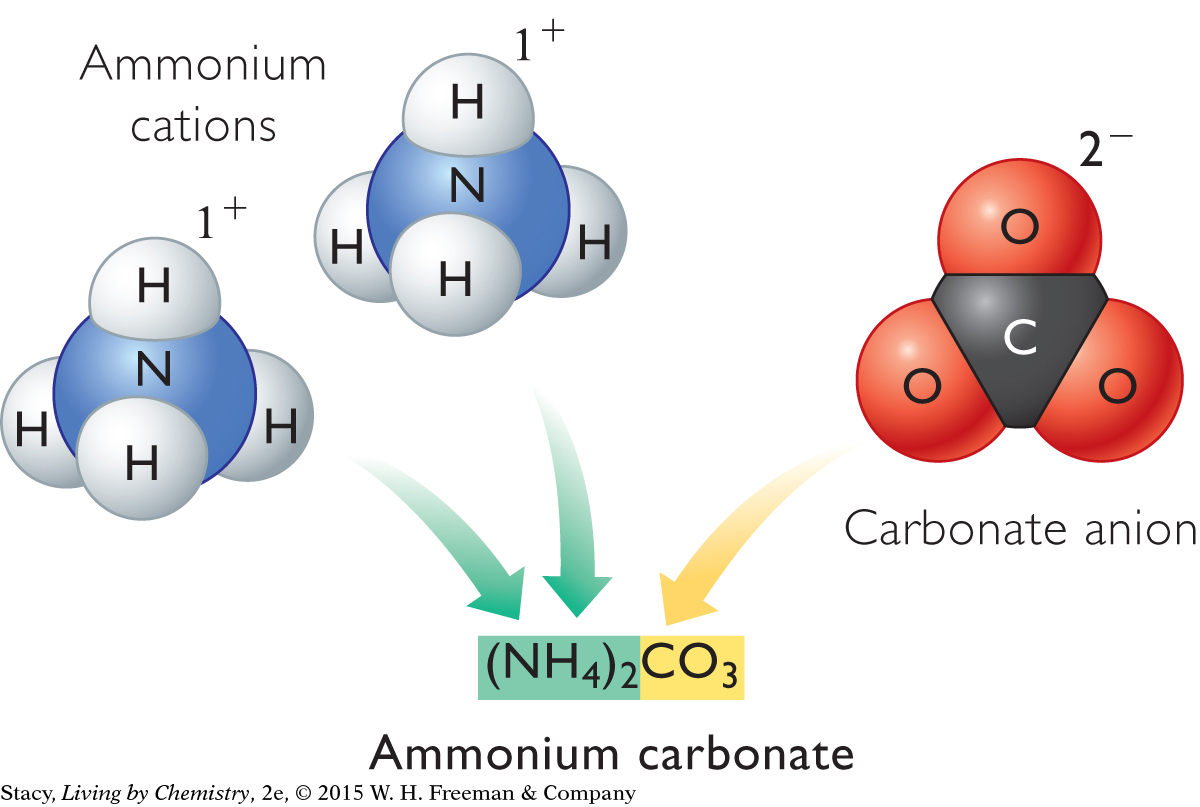
|
LESSON SUMMARY
LESSON SUMMARY
What is a polyatomic ion?
KEY TERMS
polyatomic ion
monatomic ion
Some ions are composed of more than one atom. These are called polyatomic ions. The entire cluster of atoms shares the charge on a polyatomic ion. If there are two or more of the same polyatomic ion in a chemical formula, parentheses are placed around that ion and a subscript number indicates how many ions are present. To determine the formula of a compound with polyatomic ions, you can use the rule of zero charge. Each polyatomic ion has a unique name that is used when naming the compound it is in.
Exercises
Reading Questions
What is a polyatomic ion?
How can you tell from a chemical formula if there is a polyatomic ion in a compound?
Reason and Apply
Write the name for each ionic compound listed here.
NH4Cl
K2SO4
Al(OH)3
MgCO3
Write the chemical formula for each compound listed here.
lithium sulfate
potassium hydroxide
magnesium nitrate
ammonium sulfate
Sodium cyanide, NaCN, contains a cyanide ion. What is the charge on the cyanide ion?
Calcium phosphate, Ca3(PO4)2, contains phosphate ions. What is the charge on a phosphate ion?
Which chemical formula does not represent a possible compound with sulfate, SO42–? Explain your answer.
Na2SO4
KSO4
Al2(SO4)3
CaSO4