LESSON 19: Noble Gas Envy: Ions
93
THINK ABOUT IT
Some atoms are more chemically stable than others. In other words, they don’t readily combine with other atoms to form new compounds. The atoms of the noble gas elements are considered the most chemically stable atoms on the periodic table. The most reactive elements are located just before and just after the noble gases, in Groups 1A and 7A. Perhaps the electron arrangements of these atoms are related to the atoms’ reactivity.
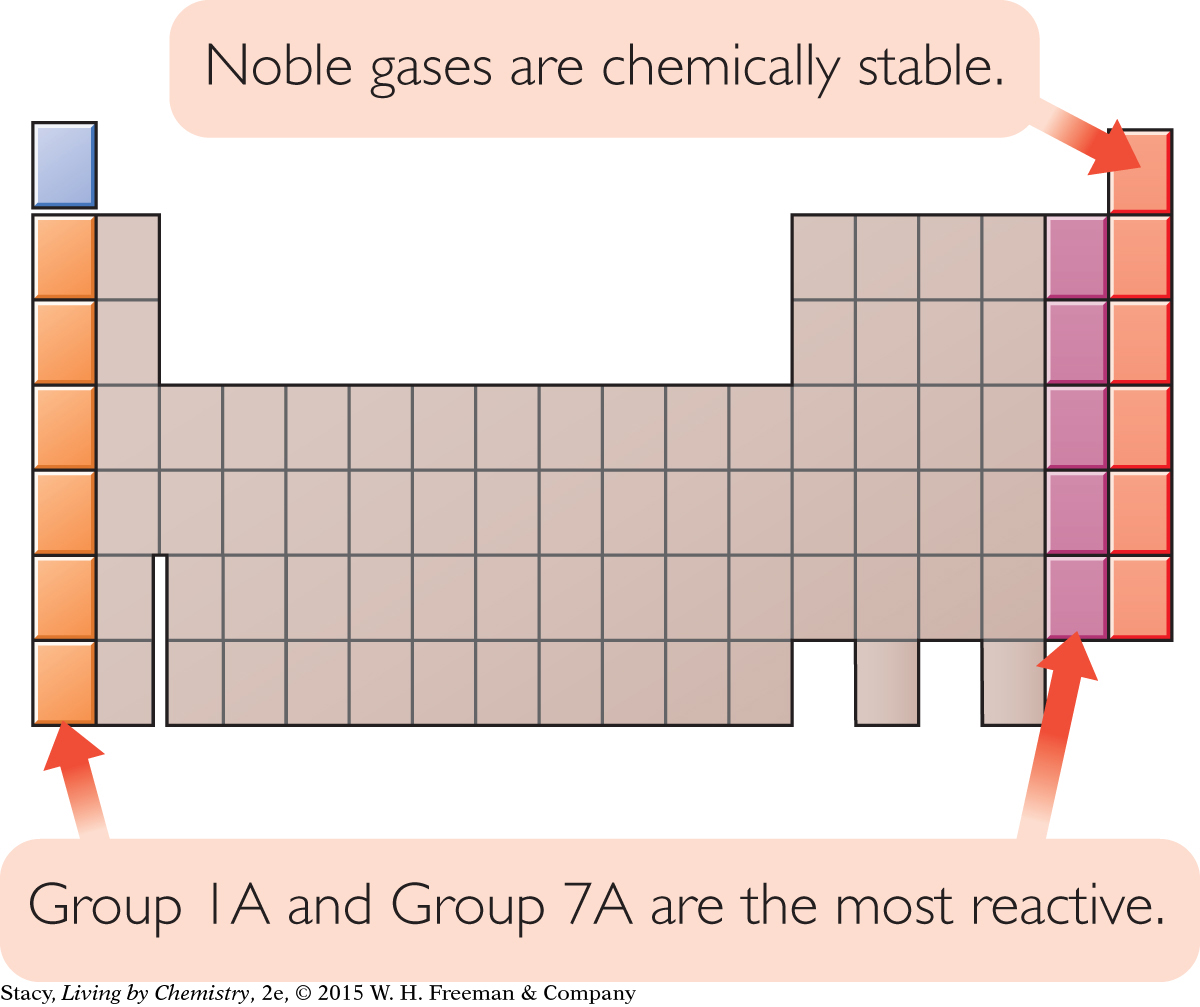
How is chemical stability related to the arrangements of electrons in atoms?
To answer this question, you will explore
Noble Gases
Noble Gas Envy
Patterns in Ion Charges
Noble Gases
EXPLORING THE TOPIC
Noble Gases
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Noble gases are so stable that they rarely form compounds. At one time it was thought that they never formed compounds with other atoms. But in 1962 a British scientist, Neil Bartlett, created xenon hexafluoroplatinate, a yellow solid, by accident. Chemists have not yet been able to create any compounds with either helium or neon. Shown here is the “light signature” of xenon.
Some elements are chemically stable, or rarely react with other elements. Others are reactive and combine readily with other elements to form compounds. What makes some elements chemically stable and others reactive?
94
Noble gases are the most chemically stable elements. Shell models for the noble gas elements helium, He, neon, Ne, argon, Ar, and krypton, Kr, are shown below. Take a moment to compare the valence electrons of each.
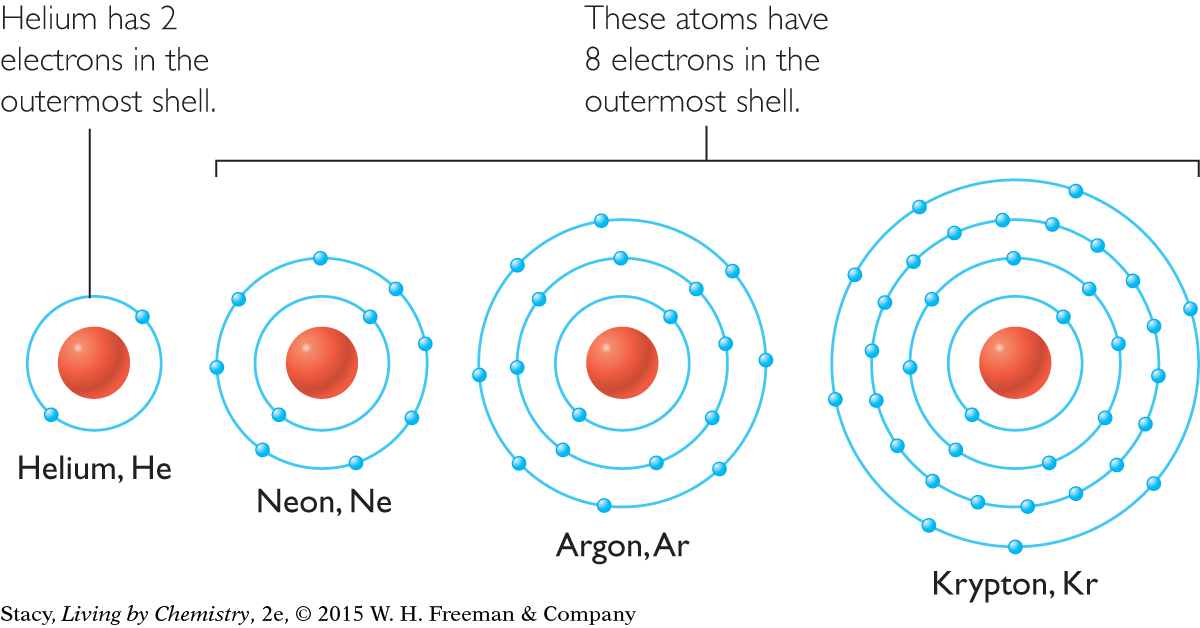
Helium has two valence electrons, which is the maximum for the first shell. The remaining noble gases each have eight valence electrons. The stability of the noble gases is associated with the number of valence electrons they have.
Noble Gas Envy
Noble Gas Envy
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Sodium and chlorine are both highly reactive. In elemental form, sodium is a soft, silvery metal, and chlorine is a greenish poisonous gas. However, they are never found in elemental form in nature. Yet they are often found as sodium chloride, which is used as table salt.

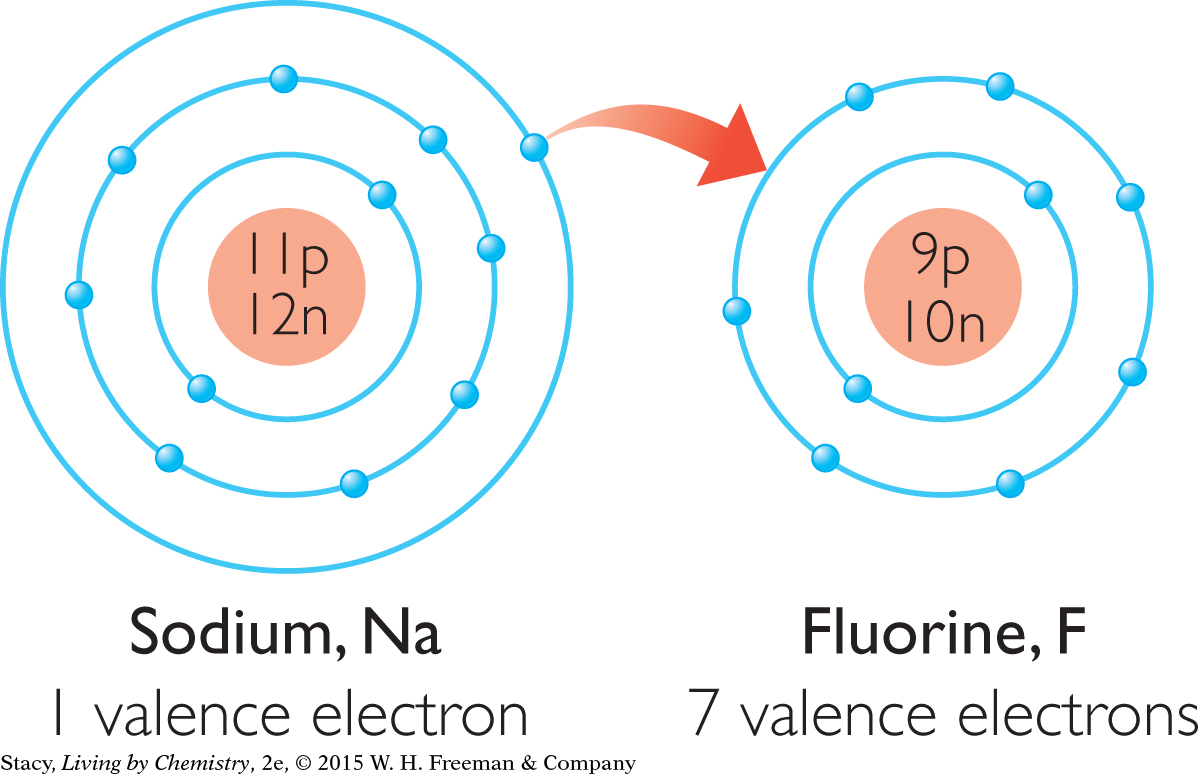
Consider two highly reactive elements, sodium, Na, and fluorine, F. The outer shell of a fluorine atom, F, has seven electrons. This is just one short of the eight electrons that neon, Ne, has in its outer shell. The outer shell of a sodium atom, Na, has one electron. This is just one more electron than neon has in total.
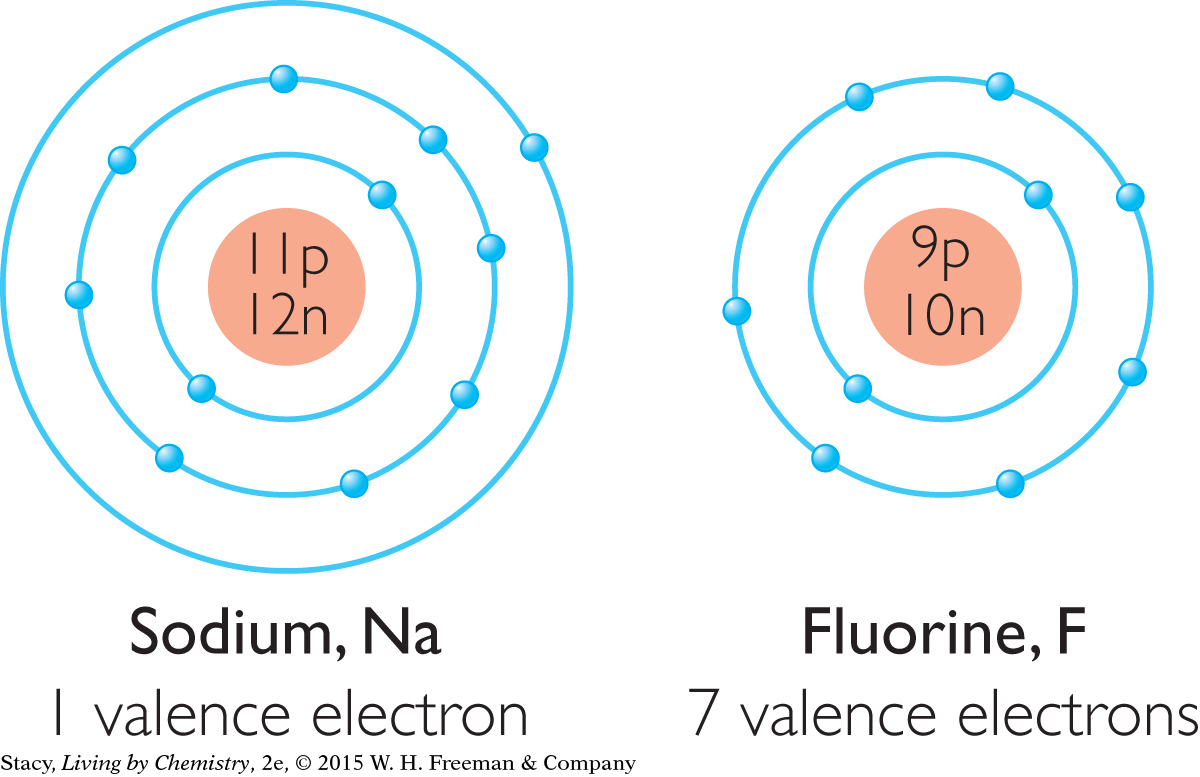
Now examine what happens to these two atoms when they combine to form a compound. Sodium, Na, gives one electron to fluorine, F.
95
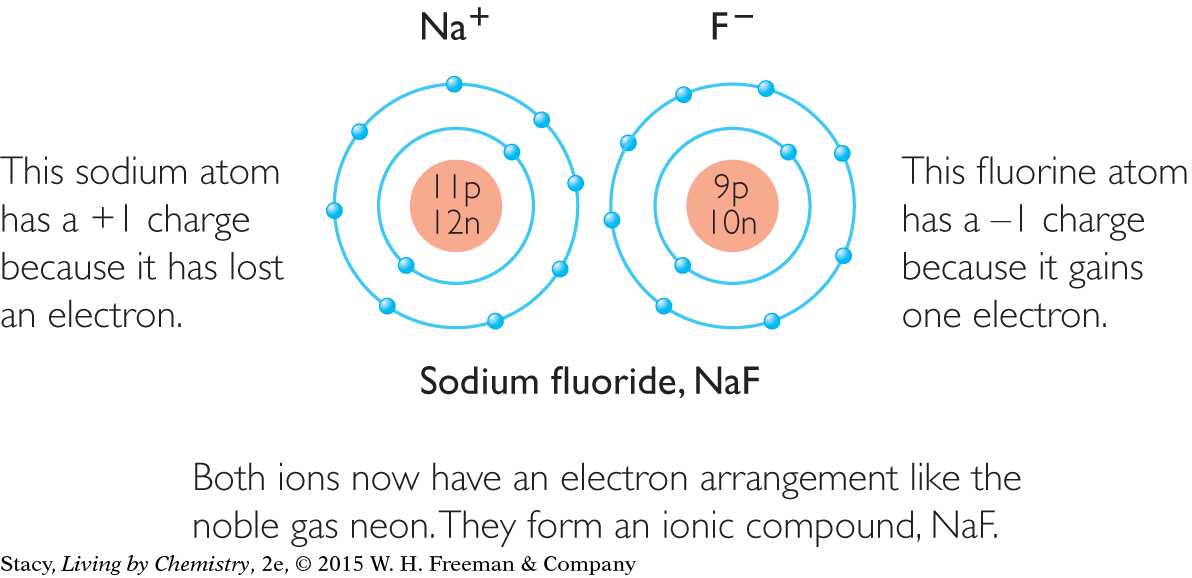
Now both atoms have an electron arrangement like that of neon, Ne.
Important to Know
When an atom loses or gains electrons, the result is a charge on the atom. The rest of the atom stays the same, and the identity of the element does not change.
The movement of an electron from one atom to another atom alters the balance of charges on both atoms. Fluorine now has more electrons than protons. Sodium has more protons than electrons. The atoms are now called ions because they possess a charge. The sodium ion has a charge of +1 and its symbol is written as Na+. The fluorine ion has a charge of –1 and its symbol is written as F–. Because Na+ and F– have opposite charges, they attract one another. So the movement of an electron from one atom to the other forms a new compound, sodium fluoride, NaF. As a result of this electron transfer, sodium atoms are now bonded to fluorine atoms.
Example
Calcium Oxide, CaO
Consider the compound calcium oxide, CaO.
Draw shell models for calcium, Ca, and oxygen, O.
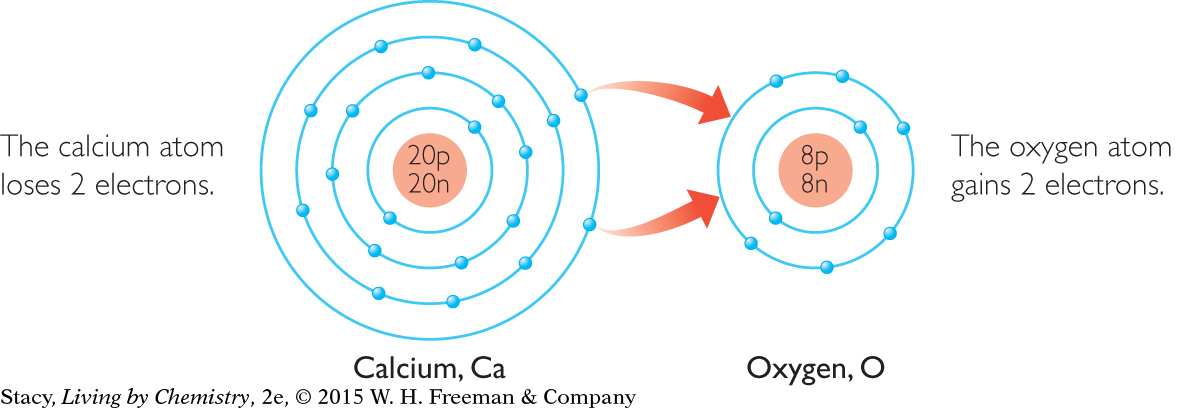
With arrows, show how electrons can be transferred so that each atom has the same electron arrangement as that of a noble gas.
What are the charges on the calcium, Ca, and oxygen, O, ions after electrons have been transferred?
Solution
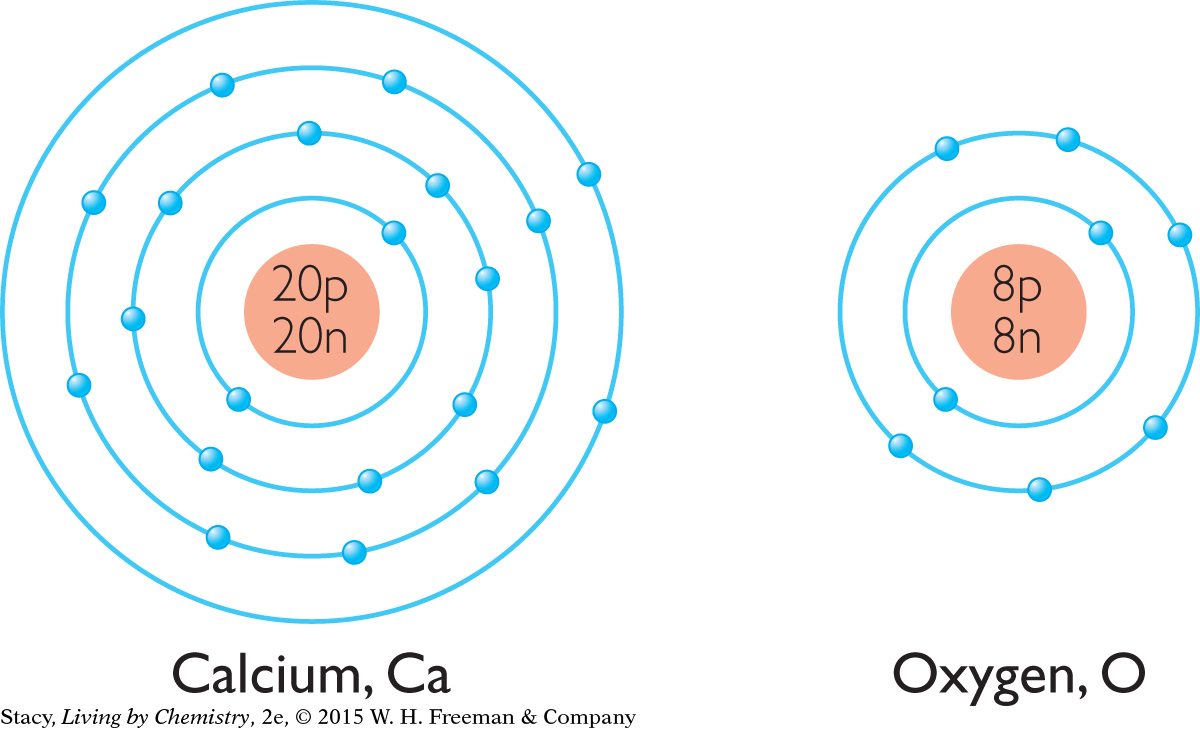
You can draw a shell model for a neutral atom, an atom that has no charge, using the element number and the element’s position on the periodic table. Calcium is in Period 4 and Group 2A, so it has four shells and two valence electrons. Oxygen is in Period 2 and Group 6A, so it has two electron shells and six valence electrons.
96
If calcium transfers two electrons to oxygen, they will both have noble gas electron configurations. The electron arrangement of oxygen now resembles that of neon, Ne. The electron arrangement of calcium now resembles that of argon, Ar.
Because calcium has lost two electrons, it now has more protons than electrons, giving it a +2 charge. The ion can be written as Ca2+. Oxygen has gained 2 electrons, so its charge is –2. Its ion can be written as O2–.
In the calcium and oxygen example, each ion has an electron arrangement that is identical to that of a noble gas. It is reasonable to assume that there is some advantage to having this sort of electron arrangement.
Noble gases are very stable the way they are, without reacting or exchanging any electrons with other elements. Apparently, other atoms can achieve some of the stability of the noble gases by exchanging electrons and becoming ions.
Patterns in Ion Charges
Patterns in Ion Charges
When atoms lose or gain electrons, they become ions, with negative or positive charges. Below is a portion of the periodic table with the various charges on the ions filled in. In every case, the ion that forms has an electron arrangement identical to the noble gas it is nearest to on the table.
Take a moment to examine the table. What patterns do you notice?
CONSUMER CONNECTION
CONSUMER
CONNECTION
Neon signs contain neon gas. When electricity is run through the gas, the electrons get excited. When they come back down to their normal energy level, they emit red light. Real neon signs glow red-orange. Signs that glow other colors are usually made of colored glass and filled with argon.

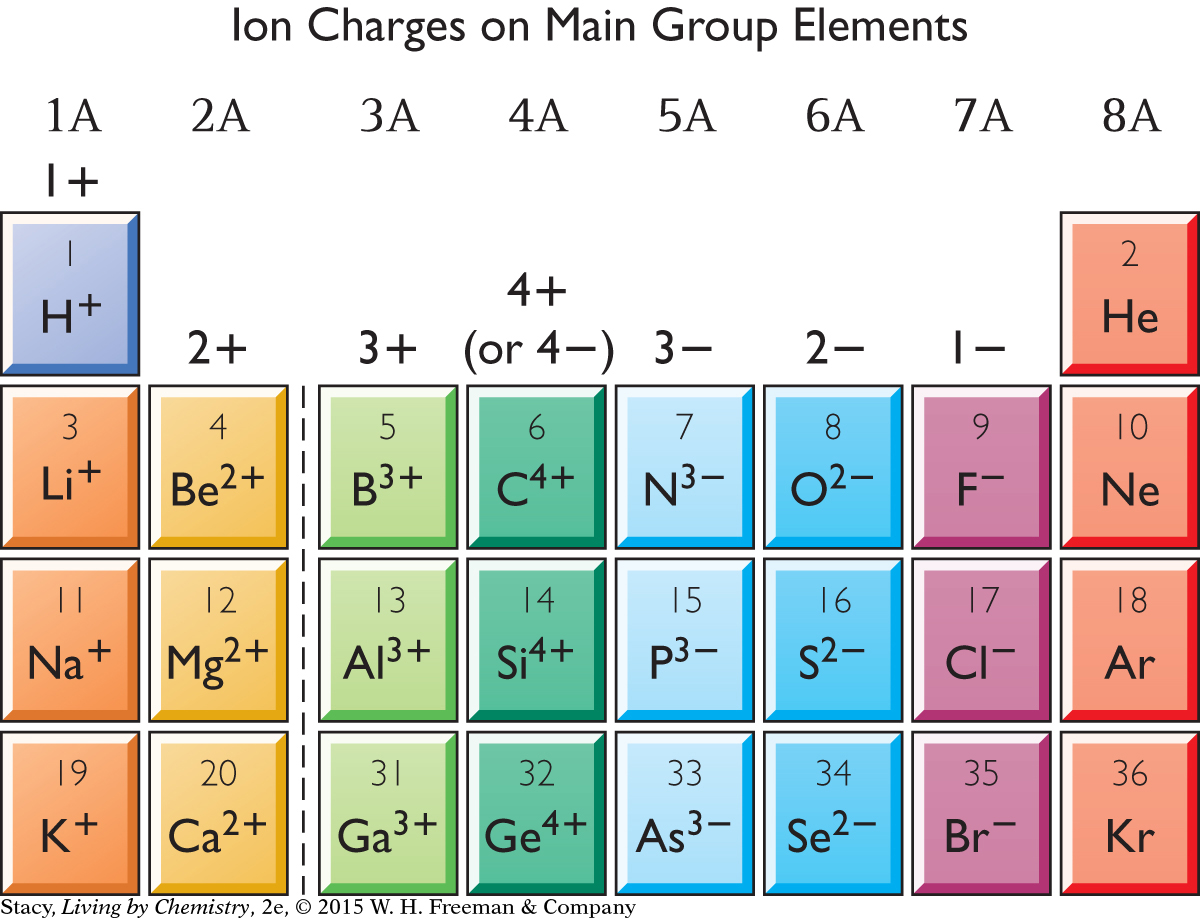
Notice that the elements on the left side of the table, which are mostly metals, tend to form ions with a positive charge. Ions with a positive charge are called cations. The valence shells of these atoms have fewer electrons in them than those of the elements on the right. So, it is easier for these elements to form compounds by giving up these few electrons.
On the other hand, the elements on the right side of the table, which are mostly nonmetals, tend to form ions with a negative charge. Ions with a negative charge are called anions.
For the first four groups of the periodic table, the ion charge is the same as the group number. For Groups 5A through 7A, the ion charge is negative and goes from –3 to –1.
LESSON SUMMARY
LESSON SUMMARY
97
How is chemical stability related to the arrangements of electrons in atoms?
KEY TERMS
ion
cation
anion
Noble gases have particularly stable atoms. This is attributed to their electron arrangements. Helium has two electrons, which is the maximum for the first shell. The remaining noble gases all have eight electrons in their outermost shells. Other atoms on the periodic table are more reactive than the noble gases. However, these reactive atoms reach greater chemical stability by gaining or losing valence electrons. Atoms tend to lose or gain electrons to attain an electron arrangement similar to that of a noble gas. Atoms do this by combining with other atoms to form new compounds. When an atom loses or gains electrons, it becomes an ion, with a charge. Ions with a positive charge are called cations. Ions with a negative charge are called anions.
Exercises
Reading Questions
Explain the difference between an anion and a cation.
Explain what is meant by noble gas envy.
Reason and Apply
How many electrons, protons, and neutrons does Li+ have?
Give two similarities and two differences between Cl and Cl–.
Give two similarities and two differences between Be and Be2+.
Which noble gas is closest to magnesium, Mg, on the periodic table? What must happen to a magnesium atom for it to have an electron arrangement similar to that of a noble gas?
Which noble gas is closest to sulfur, S, on the periodic table? What must happen to a sulfur atom for it to have an electron arrangement similar to that of a noble gas?
List four ions that have the same number of electrons as neon, Ne.
List four ions that have the same number of electrons as argon, Ar.
What charge would an arsenic, As, ion have?
What is the symbol of an ion with 22 protons, 24 neutrons, and 18 electrons?
When chlorine gains an electron to become a chloride ion with a –1 charge, it ends up with the electron arrangement of argon. Why doesn’t it become an argon atom?
Explain why the elements on the right side of the periodic table gain electrons instead of losing them.
What periodic patterns do you notice for the charges on the ions?
Which of these ions have the correct charge? Choose all that apply.
Na2+
Li+
Al4+
Ca2+
Ga3+
Which of these ions have the same number of electrons as S2–? Choose all that apply.
Cl–
Ca2+
Na+
O2–
P3–