Featured LAB: Classifying Substances
Featured LAB
Classifying Substances

Materials
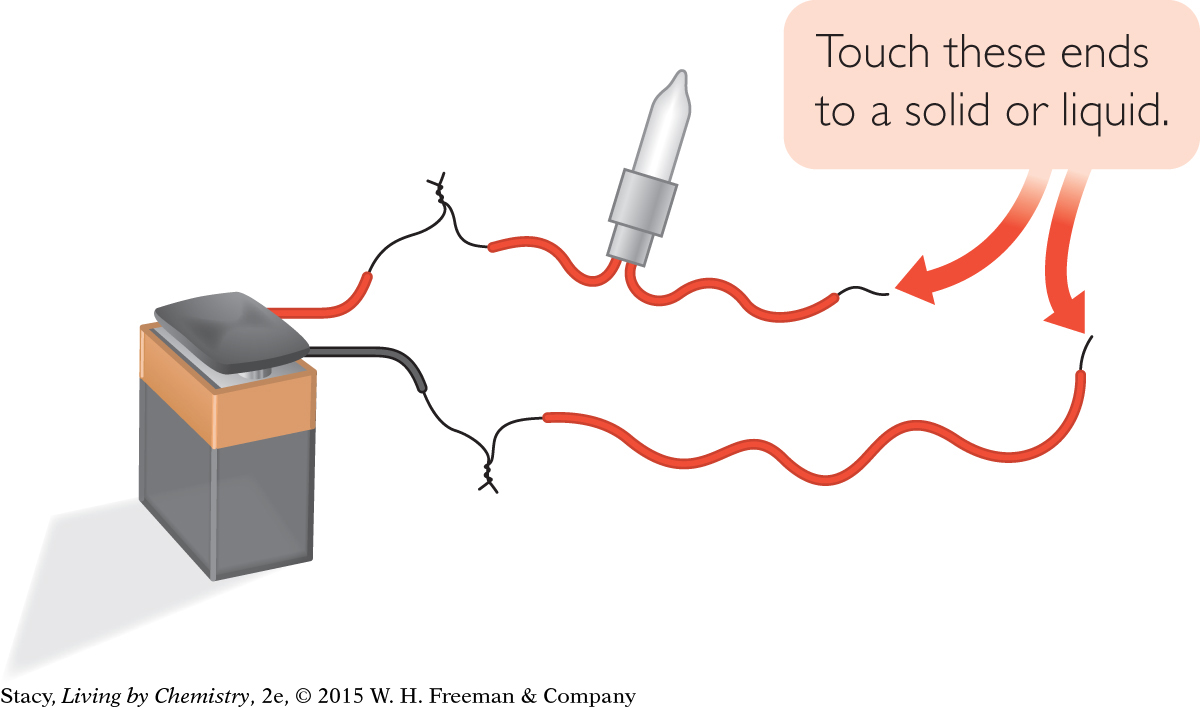
bulb with wires
9-volt battery with snap connector
wire with stripped ends
100 mL beakers
paper clips
salt, NaCl
sand, SiO2
paraffin wax, C20H42
calcium chloride, CaCl2
copper, Cu
copper (II) sulfate, CuSO4
aluminum foil, Al
sucrose, C12H22O11
distilled water, H2O
Purpose
To investigate the properties of substances.
Predictions and Data
Predict whether each substance will conduct electricity and whether it will dissolve in water. Make a table for your predictions and data.
Procedure
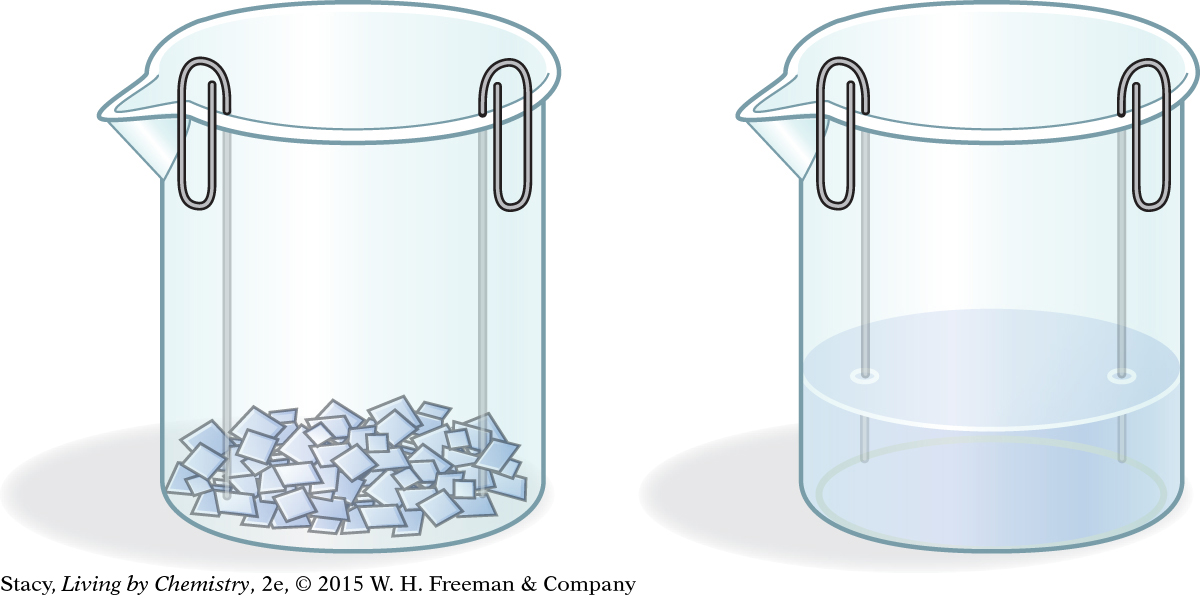
Assemble your conductivity tester as shown.
Take your conductivity tester to each station. Test the pure substance for conductivity.
Next, observe the substance in water. Did the substance dissolve?
Finally, test the substance mixed with the distilled water in the second beaker for conductivity. Record your results in a data table.
Analysis
Group the substances according to their properties. How do your results compare with your predictions? What do the substances in each group have in common? Summarize your findings in a paragraph.