LESSON 27: Electrons on the Move: Electroplating Metals
THINK ABOUT IT
Metals are important in our lives. They are used for everything from electrical wiring, jewelry, and soda cans, to cars, airplanes, and bridges. Most metals are dug out of the ground as ionic compounds. In ionic compounds, the metal atoms are bonded to nonmetal atoms. Through the ages, people have struggled to extract the pure metals from these compounds by figuring out ways to separate the metal atoms from the nonmetal atoms.
How can you extract an element from a compound?
To answer this question, you will explore
The Quest for Precious Metals
Electroplating Elemental Metals
The Quest for Precious Metals
EXPLORING THE TOPIC
The Quest for Precious Metals
HISTORY CONNECTION
HISTORY
CONNECTION
The first metal tools, implements, and weapons were made from copper. Gold, copper, silver, tin, lead, iron, and mercury are often referred to as the Metals of Antiquity. For some 7000 years, these seven metals were the only ones in widespread use. These metals were known to the Mesopotamians, Greeks, Egyptians, and Romans.

Sometime around 6000 B.C.E., metals came into widespread use. Before that, most tools and implements were made from earth, bone, wood, and stone. The first metals to be discovered were gold, copper, and silver. Later, lead, tin, iron, and mercury were added to the list. Most of these metals are not readily found in nature in pure form.

The first metals that were used were the ones people stumbled upon in their pure form in the earth. Then, people discovered that, using heat, they could extract metals from other compounds dug out of the earth. For example, if iron (III) oxide, Fe2O3, is heated in the presence of charcoal, the oxygen in the compound is removed as CO2 gas. This leaves behind solid iron, Fe. This process requires very high temperatures.
For nearly 7000 years, only a handful of metals was in widespread use. Other metals, such as aluminum, Al, could not be extracted easily by heating and remained unavailable.
Electroplating Elemental Metals
Electroplating Elemental Metals
USING ELECTRICITY TO EXTRACT METALS
Over time, the construction of better furnaces has resulted in the extraction of a variety of metals. More recently, it was discovered that electricity could be used to extract metals from compounds. Recall that electrons are transferred from metal atoms to nonmetal atoms in ionic compounds. Scientists discovered they could “give” electrons back to the metal ions, re-forming the neutral metal atoms once again. For example, copper metal can be extracted from a solution of copper sulfate by running an electrical current through the solution. A simple setup is shown here.
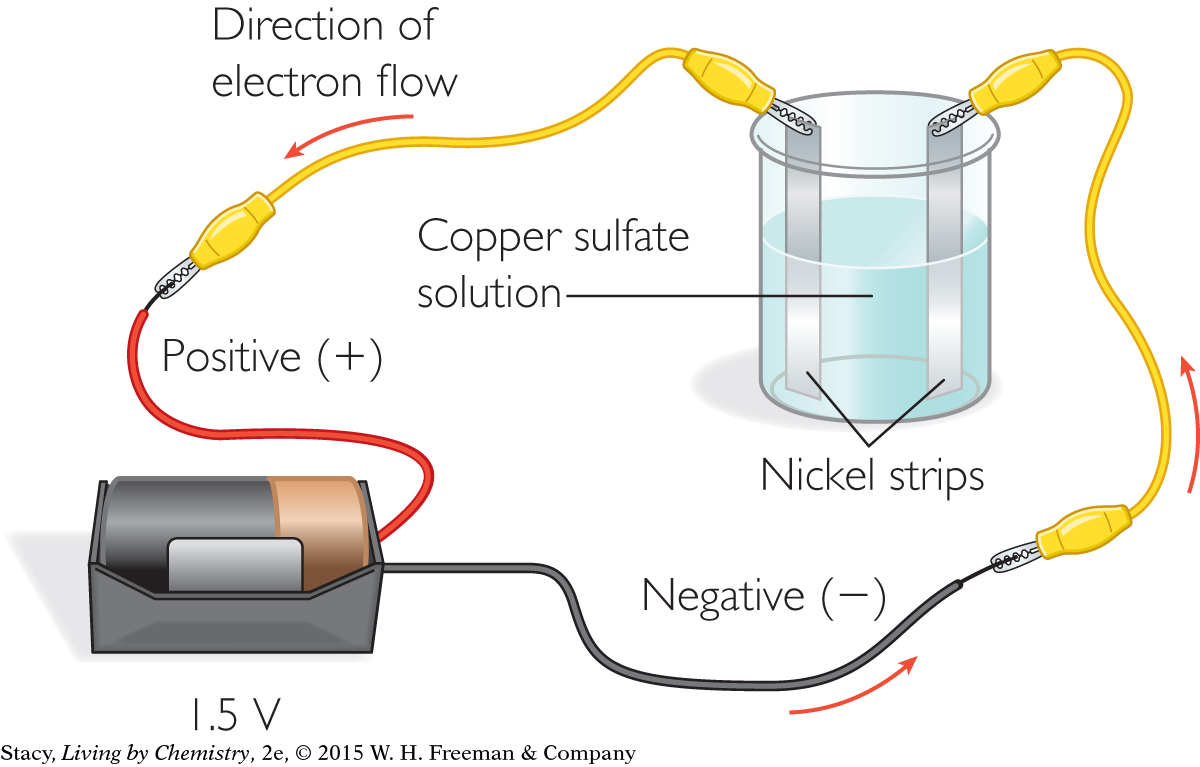
Once the battery is hooked up, one nickel strip has a positive charge and the other has a negative charge. In this electrical circuit, the electrons move from the negative terminal of the battery to the negatively charged nickel strip. Electrons also move from the other nickel strip toward the positive terminal of the battery. Ions moving in solution complete the circuit.
The positive copper ions, Cu2+, are attracted to the nickel strip with the negative charge. Electrons are added to the copper cations in solution. Each copper ion that gains enough electrons becomes elemental copper. When the copper cations become elemental copper atoms, they come out of solution and coat the negatively charged nickel strip. This process is called electroplating.
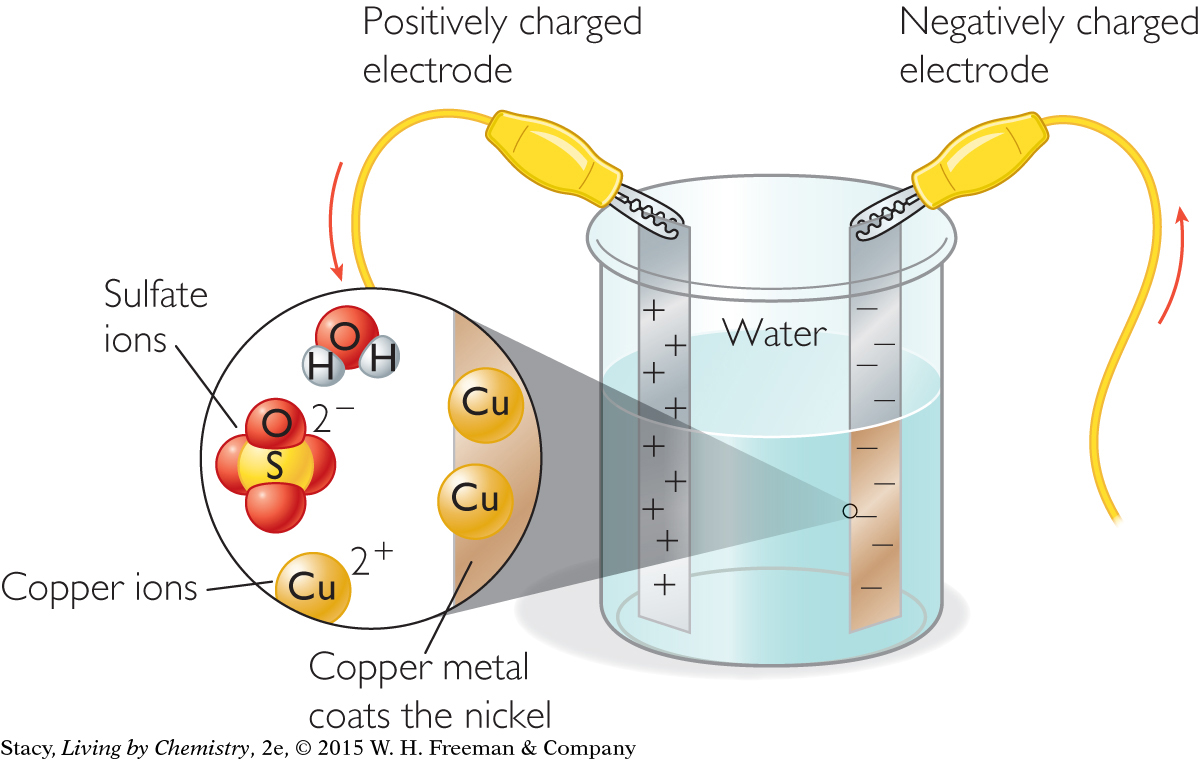
When the experiment shown is completed, the negatively charged nickel becomes coated with the pure copper metal.
You can reverse the process and produce metal cations from metal atoms. The battery has a positive and a negative terminal. By switching the wires in the test setup, you can change the direction the electrical charge moves. This causes the reaction to reverse. The copper coating comes off of the first nickel strip and reenters the solution. Copper from the solution plates onto the other nickel strip.
ELECTROPLATING GOLD
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Decorative chrome plating is used on some cars and motorcycles.

Is it possible to design an apparatus similar to the one in the Lab: Electroplating Metals to extract gold atoms from ionic compounds containing gold ions? Yes, by all means. However, gold is one of those few elements that is commonly found in nature in its elemental form. Gold compounds are seldom found in nature. This is because gold is fairly stable as it is. So harvesting gold atoms from gold ions is not the best way to make a pile of gold bars.
However, electroplating techniques can be used to make a little bit of gold go a long way. Using electroplating or other plating techniques, less expensive metals can be covered with thin layers of gold atoms.

Today, electroplating is an extremely important industrial process. For example, aluminum metal has been in widespread use only recently because chemists finally found a way to extract it from ionic compounds using electrochemical methods. Electroplating is also used to coat electronic parts in computers.
Example
Plating Gold from AuCl3
Suppose that you have a sample of AuCl3(s).
What is the charge on the Au ions in AuCl3?
What is the chemical name of AuCl3?
Do you expect AuCl3 to conduct electricity as a solid? Explain your thinking.
Do you expect AuCl3 to dissolve in water? Explain your thinking.
Describe how you can extract gold from AuCl3.
Solution
You need to find the charge on the main group element first. Cl is in Group 7B in the periodic table, which means that it has seven valence electrons. So, Cl needs to gain an electron, which will make it Cl–. The Au becomes Au3+ because the charges in an ionic compound must add up to zero.
The gold has a +3 charge. So, the name is gold (III) chloride.
AuCl3 is an ionic solid. Ionic solids do not conduct electricity. However, if you dissolve AuCl3 in water, the solution will conduct electricity.
Because AuCl3 is ionic, you can assume it will dissolve in water, forming Au3+ and Cl– ions.
Gold can be plated onto a piece of nickel by using a battery to move electrons through a solution containing AuCl3.
LESSON SUMMARY
LESSON SUMMARY
How can you extract an element from a compound?
KEY TERM
electroplating
Most metal atoms are found in nature combined with other atoms, in the form of ionic compounds. Some metals can be extracted from ionic compounds through heating. Elements can also be extracted from ionic compounds by using electricity to move electrons between atoms. Using electroplating, you can create a variety of substances that could be considered “as good as gold.”
Exercises
Reading Questions
Describe three ways in which people have managed to obtain pure metals to use for making tools and other objects.
Explain how to plate copper metal onto a nickel strip. Write a simple procedure and draw a labeled sketch showing how to carry it out.
Explain how you can remove a metal coating from an object.
Reason and Apply
Lab Report Complete a lab report for the copper plating experiment. In your report, give the title of the experiment, purpose, procedure, observations, and conclusions.
Explain how the copper plating experiment supports the claim that Cu2+ is just a Cu atom that is missing two electrons.
If you measure the mass of the nickel strip before and after plating copper, do you expect the mass to change? Explain your thinking.
Suppose that a classmate claimed that the nickel in the electroplating lab changed into copper. Provide an argument to prove that this is not true.