LESSON 34: Create a Smell: Ester Synthesis
175
THINK ABOUT IT
Body odor, stinky shoes, moldy carpets, dog odors, musty basements, bad breath—these are just a few of the smells that we consider unpleasant. But the structures of these molecules are not so different from the structures of sweet-smelling substances. Molecules that have a carboxyl functional group are very similar to molecules that have an ester functional group. Perhaps there is a way to change one type of molecule into the other.
How can a molecule be changed into a different molecule by using chemistry?
To answer this question, you will explore
Transforming Smells
Chemical Reactions
Chemical Synthesis
Transforming Smells
EXPLORING THE TOPIC
Transforming Smells
CONSUMER CONNECTION
CONSUMER
CONNECTION
The putrid-smelling compound butyric acid is formed when butter goes bad. In fact, butyric means “from butter.” Butyric acid is also found in Parmesan cheese and vomit.

Every year, consumers spend millions of dollars to purchase products that will deal with unwanted odors. Often, these products do nothing more than cover up a foul smell with a pleasing smell. But some products use chemistry to change the molecular structure of the smelly molecules.
Butyric acid, C4H8O2, is a carboxylic acid with a putrid smell. However, the difference between this molecule and a sweet-smelling molecule is minimal. Butyric acid can be chemically changed into a sweet-smelling ester.
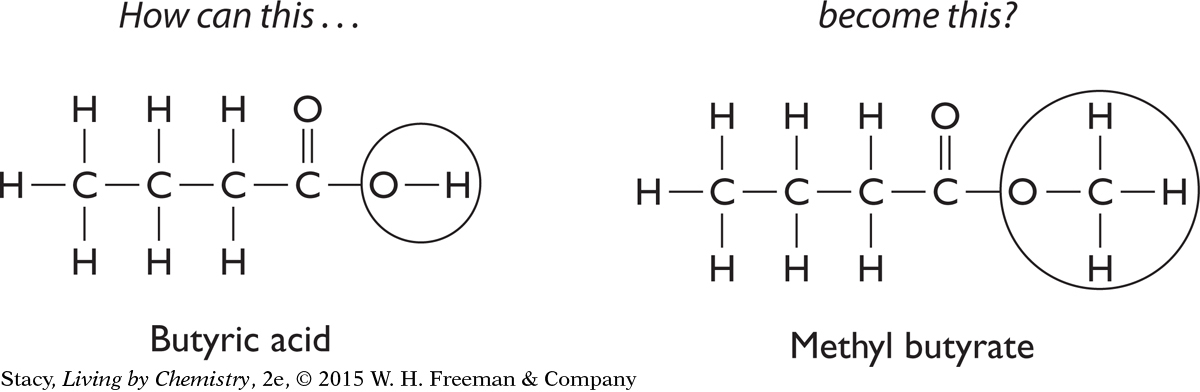
You can see that to accomplish this task, you would have to somehow remove the hydrogen atom that is attached to an oxygen atom in the butyric acid molecule and attach in its place a carbon atom with three hydrogen atoms. This procedure is possible in a chemistry lab.
Chemical Reactions
Chemical Reactions
176
CONSUMER CONNECTION
CONSUMER
CONNECTION
An entire industry is devoted to the creation of fragrances and flavors for use in our foods and commercial products. Some companies even manufacture room odorizers to inject a pleasant aroma into public environments or businesses. These aromas include the smell of freshly brewed coffee, baking bread, or an evergreen forest. The idea is to create environments that evoke pleasant memories or experiences for the user—and to stimulate the appetite or the pocketbook.

In the ester synthesis lab, you mixed some substances together with a catalyst and heated them, following a specific set of instructions. The result was a molecule that no longer smelled bad.
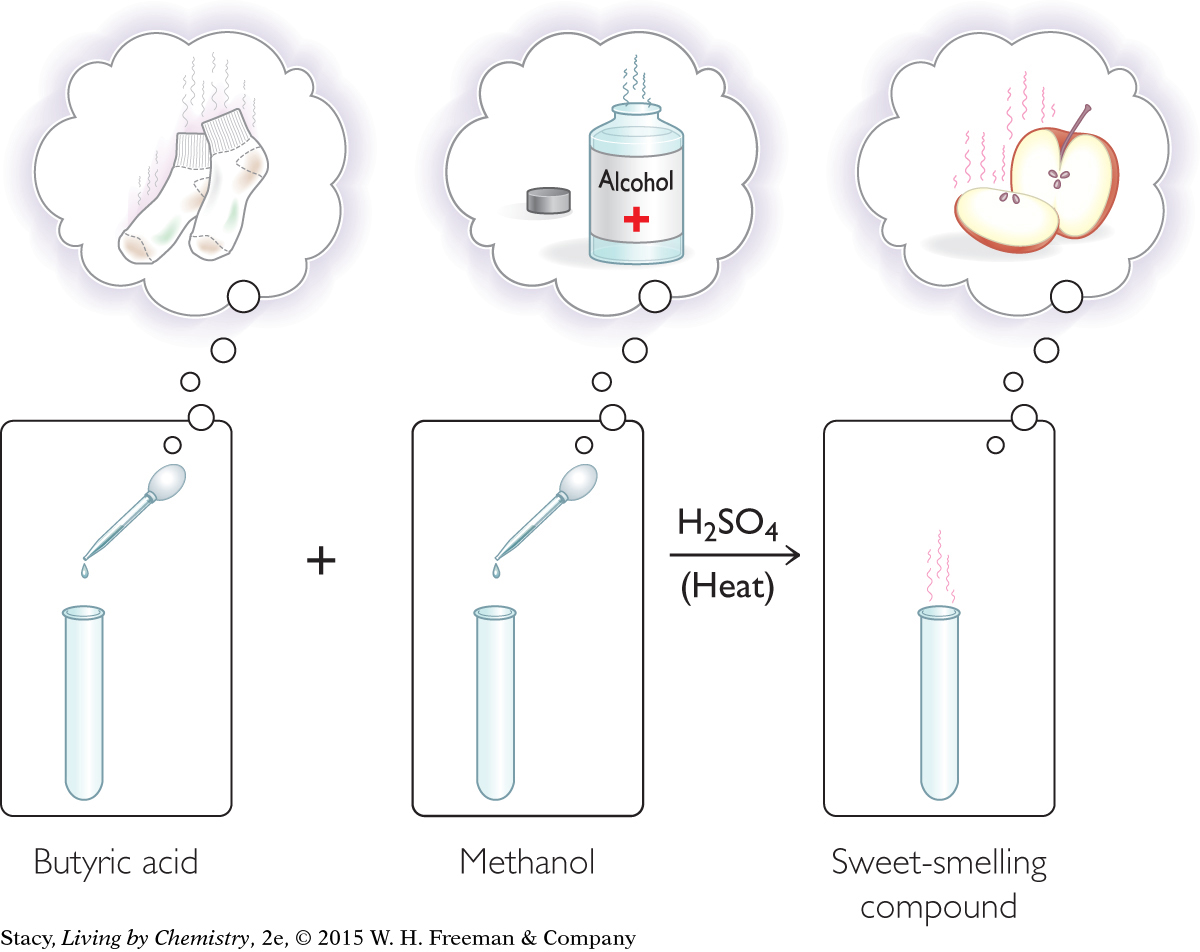
When butyric acid and methanol are mixed and heated, no change is visible to the eye. However, your nose tells you that a dramatic change in properties has occurred. The foul smell of sweaty socks disappears and the sweet smell of apples fills the room.
The change in smell is clear evidence that a chemical reaction has occurred. A chemical reaction is the process by which matter is changed so that new substances are formed. For new substances to form, the atoms must rearrange. Some bonds between atoms must be broken and new bonds must be formed. The result of a chemical reaction is a new compound with entirely different properties. In this case, one of the new properties is a different smell.
The detection of a sweet-smelling compound is evidence that you have made an ester. In fact, methyl butyrate smells like apples. In Lesson 35: Making Scents, you will examine this transformation in greater detail.
Chemical Synthesis
Chemical Synthesis
Chemists often work in the laboratory with the goal of producing a specific compound. This process is called synthesis. Synthesis is the process of producing a chemical compound, usually by combining two simpler compounds. Many of the products that we have come to depend on are products that chemists have synthesized, like plastics, fabrics, cosmetics, deodorants, cleaning products, vitamins, and medications.
Sometimes chemists work to synthesize a molecule that is very rare or hard to find. For instance, many lifesaving, anti-cancer compounds have been discovered in plant and animal life in the rainforests of South America. It may be difficult to harvest these compounds or find enough of a substance to treat the many patients who are in need. Chemists study the structure of a compound like this and work to create it in the laboratory. Most of the medicines in use today are synthesized in laboratories.
177

Sometimes the point of synthesis is to create a new molecule that is not found in nature. All of the plastics that we have in our lives are synthesized from small molecules that have been strung together through chemical reactions. These compounds are valued for their amazing properties. Many plastics are durable, flexible, and easily molded into a variety of useful shapes—everything from shoes to computer keyboards.
LESSON SUMMARY
LESSON SUMMARY
How can a molecule be changed into a different molecule by using chemistry?
KEY TERMS
chemical reaction
synthesis
To transform a putrid-smelling molecule into a sweet-smelling molecule, it is necessary to transform a carboxylic acid molecule into an ester molecule. This transformation requires a chemical reaction in which bonds between certain atoms are broken and new bonds are made. The result of any chemical reaction is a different compound or compounds than the compound you started with. And when the functional group changes, the smell changes. Chemists control chemical reactions in the laboratory to synthesize specific molecules with valuable properties.
Exercises
Reading Questions
What would you do to cause an alcohol and an acid to react?
What is chemical synthesis?
Reason and Apply
In class, why do you think the foul smell of butyric acid disappeared when you mixed it with methanol and heated the mixture in the Lab: Ester Synthesis?
The structural formulas for the molecules used in the Lab: Ester Synthesis are given in the table. Identify the functional groups.
178
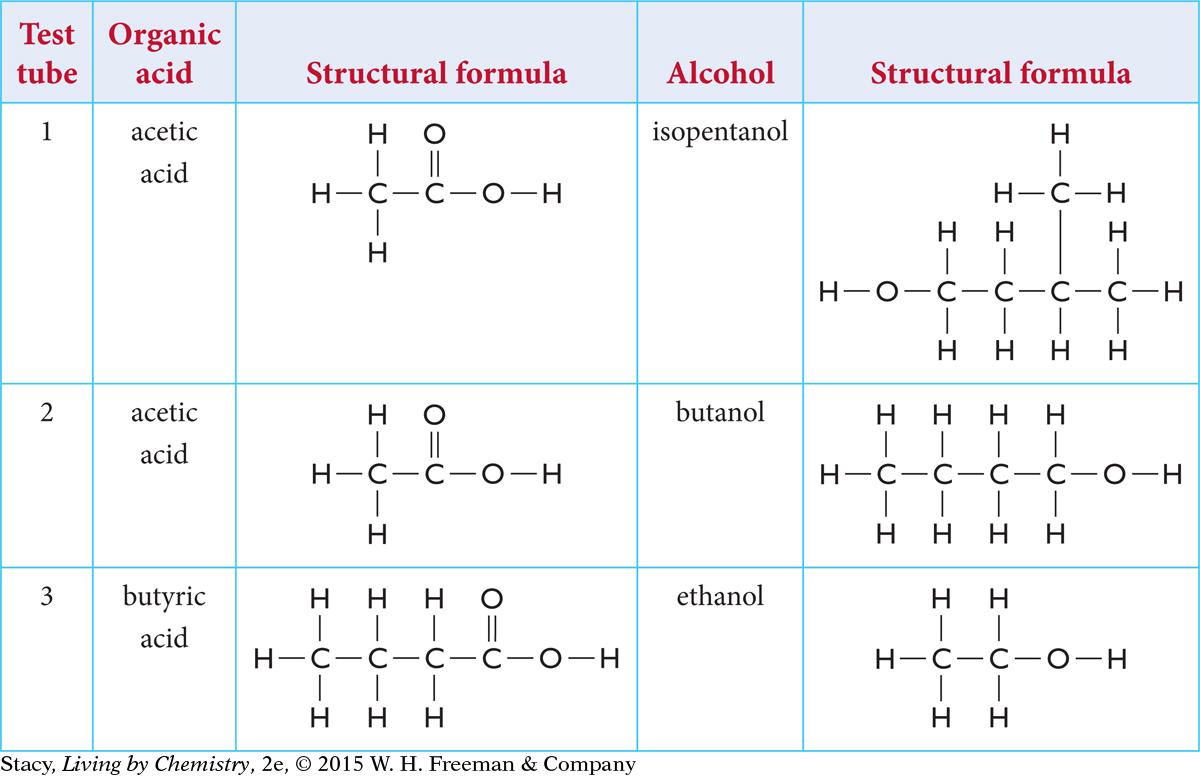
Explain how you can convert acetic acid into an ester. Be specific about how the molecule needs to change.
Lab Report Write a lab report for the Lab: Ester Synthesis. In your report, include the title of the experiment, purpose, procedure, observations, and conclusions.
