LESSON 35: Making Scents: Analyzing Ester Synthesis
THINK ABOUT IT
CAREER CONNECTION
CAREER
CONNECTION
A flavorist’s job is to create or re-create flavors. To create flavors for foods, toothpaste, chewable medications, lipgloss, and other products, the flavorist will use his or her knowledge of the available chemical ingredients to create a flavor compound. The flavorist may test many different compounds until the right flavor is found.

When you heat a mixture of a carboxylic acid and an alcohol, a chemical reaction takes place. You can smell the outcome. A really putrid-smelling substance is transformed into one that smells sweet. An ester has been produced. But what has happened to the original substances that were mixed together?
What happened to the molecules during the creation of a new smell?
To answer this question, you will explore
Chemical Equations
The Role of the Catalyst
Ester Synthesis
Chemical Equations
EXPLORING THE TOPIC
Chemical Equations

Chemists use chemical equations to keep track of changes in matter. These can be certain physical changes or changes due to chemical reactions. A chemical equation usually uses chemical formulas to describe what happens when substances are mixed together and new substances with new properties are formed. In class, you used structural formulas to describe what happened to the molecules that were combined.
Consider what happens if you add butyric acid to ethanol and create a pineapple smell. A description of the reaction is given here. Each of these substances consists of a collection of molecules that are structurally different. The structural formulas for the molecules in each of the substances are also shown along with their chemical formulas.
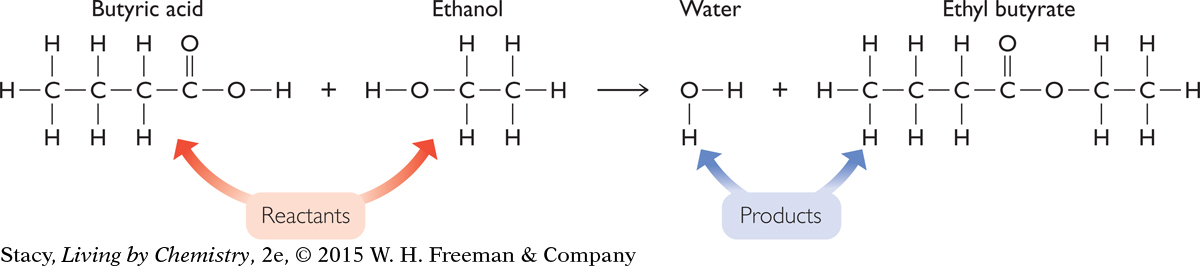
In a chemical reaction, the substances that are mixed together are called the reactants. The new substances that are produced are called the products.
The reactants in this chemical reaction are butyric acid and ethanol. One product that is easily detected by smell is ethyl butyrate. However, this is not the only product. Although it is difficult to observe with the five senses, water is also a product of this chemical reaction. The chemical equation helps to make this clear. The chemical equation shows that in a chemical reaction, no matter is created or destroyed.
Big Idea
Big Idea
Matter cannot be created or destroyed. Matter is conserved.
The next illustration highlights the areas of the reactant molecules that change during the chemical reaction. You can see that a hydroxyl, –OH group, breaks off from the butyric acid molecule. In addition, the H–O bond in the ethanol molecule must also break. A new bond forms as the larger molecular pieces come together to form ethyl butyrate.
Finally, you can see that the H– and –OH pieces that are left over combine to form H2O, water.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Esters are found in a number of household products, from cleansers and air fresheners to hand lotions and shampoos. Their main role is to make a product smell a certain way, although some are detergents as well.

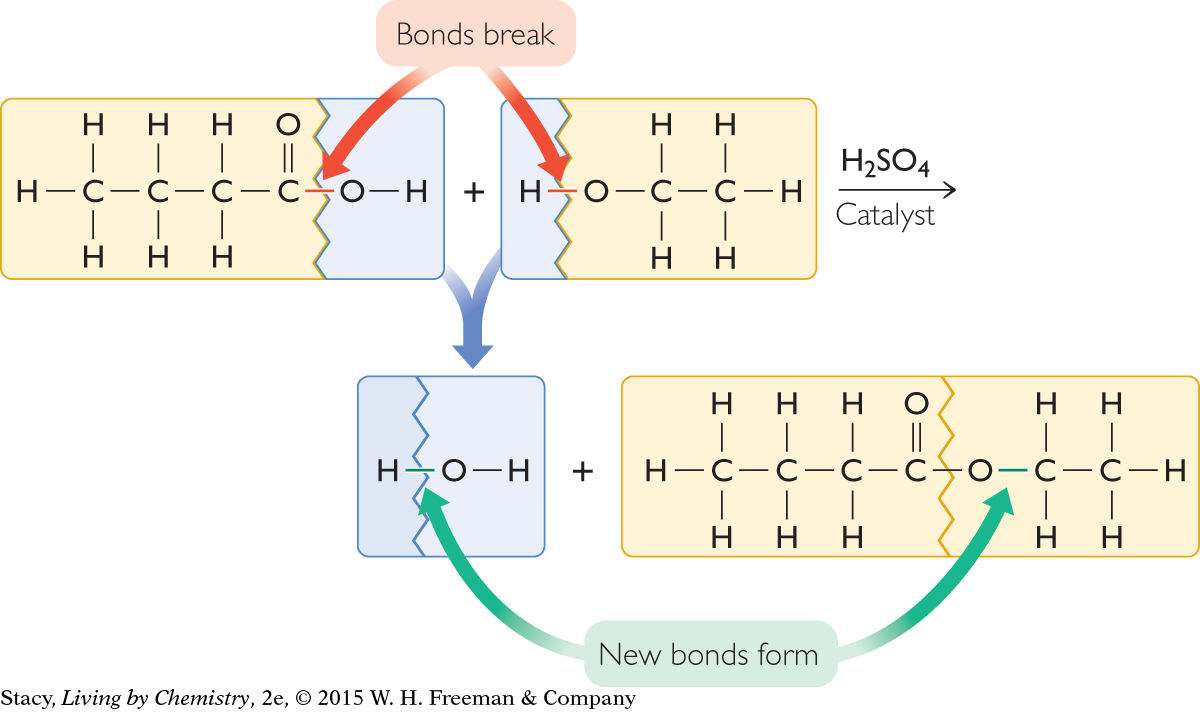
For every butyric acid molecule that reacts with an ethanol molecule, one molecule of water is produced along with one molecule of ethyl butyrate.
The complete chemical equation for this chemical reaction is
C4H8O2 + C2H6O → H2O + C6H12O2
There are the same number of each type of atom on both sides of the equation. All of the matter is accounted for.
The Role of the Catalyst
The Role of the Catalyst
CONSUMER CONNECTION
CONSUMER
CONNECTION
A catalyst is often used in an automobile. It is located in the catalytic converter in the muffler of the car. Its job is to help break down some of the harmful products in engine exhaust. Platinum is one of the elements used in catalytic converters.

There’s one more ingredient in the chemical reaction that we have not yet discussed. When you try to carry out this reaction in a lab, you will find that simply mixing butyric acid and ethanol does not produce ethyl butyrate. In fact, nothing happens. To make the chemical reaction happen, it is necessary to add sulfuric acid, H2SO4, and heat the mixture.
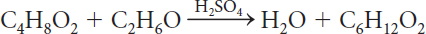
The sulfuric acid does not get used up during the reaction. It is still present at the end. In this reaction, the sulfuric acid is simply helping the reaction along. Chemists refer to a substance that assists a chemical reaction as a catalyst. A catalyst speeds up a reaction, but the catalyst itself is not consumed by the reaction. In chemical equations, a catalyst is written above the arrow.
Ester Synthesis
Ester Synthesis
Many different acids and alcohols can be brought together to form an ester and water. The general description of this reaction is
acid + alcohol → water + ester
Take another look at the illustration on page 180 showing the bonds breaking. It is not necessary for every single bond in the acid and alcohol molecules to break apart to form the ester. All of the C–H bonds remain connected during this reaction.
During chemical reactions, the major changes in the molecules often occur where the functional groups are located. The part of the molecule that is easiest to change is the functional group. This makes sense with what you already know: The functional group is directly related to the properties of a molecule.
LESSON SUMMARY
LESSON SUMMARY
What happened to the molecules during the creation of a new smell?
KEY TERMS
chemical equation
reactant
product
catalyst
When chemical reactions occur, new compounds with different properties and structures are produced. The substances that are combined are called the reactants. The substances that are produced are called the products. When chemical reactions occur, bonds break and new bonds form. Chemical equations use chemical formulas to track the changes that occur during chemical reactions. Because matter is conserved, all atoms are accounted for in a chemical equation. Some chemical reactions require a catalyst to help them get started or proceed more rapidly. A catalyst is not consumed by the chemical reaction it is assisting.
Exercises
Reading Questions
Explain why converting a carboxylic acid to an ester might be useful.
Describe what happens during a chemical reaction.
What is a catalyst?
Reason and Apply
Write a paragraph answering the question, “How can scientists use chemistry to create compounds with specific smells?” Include evidence to support your answer.
Below are the structural formulas for four esters. Write the correct molecular formula for each one.
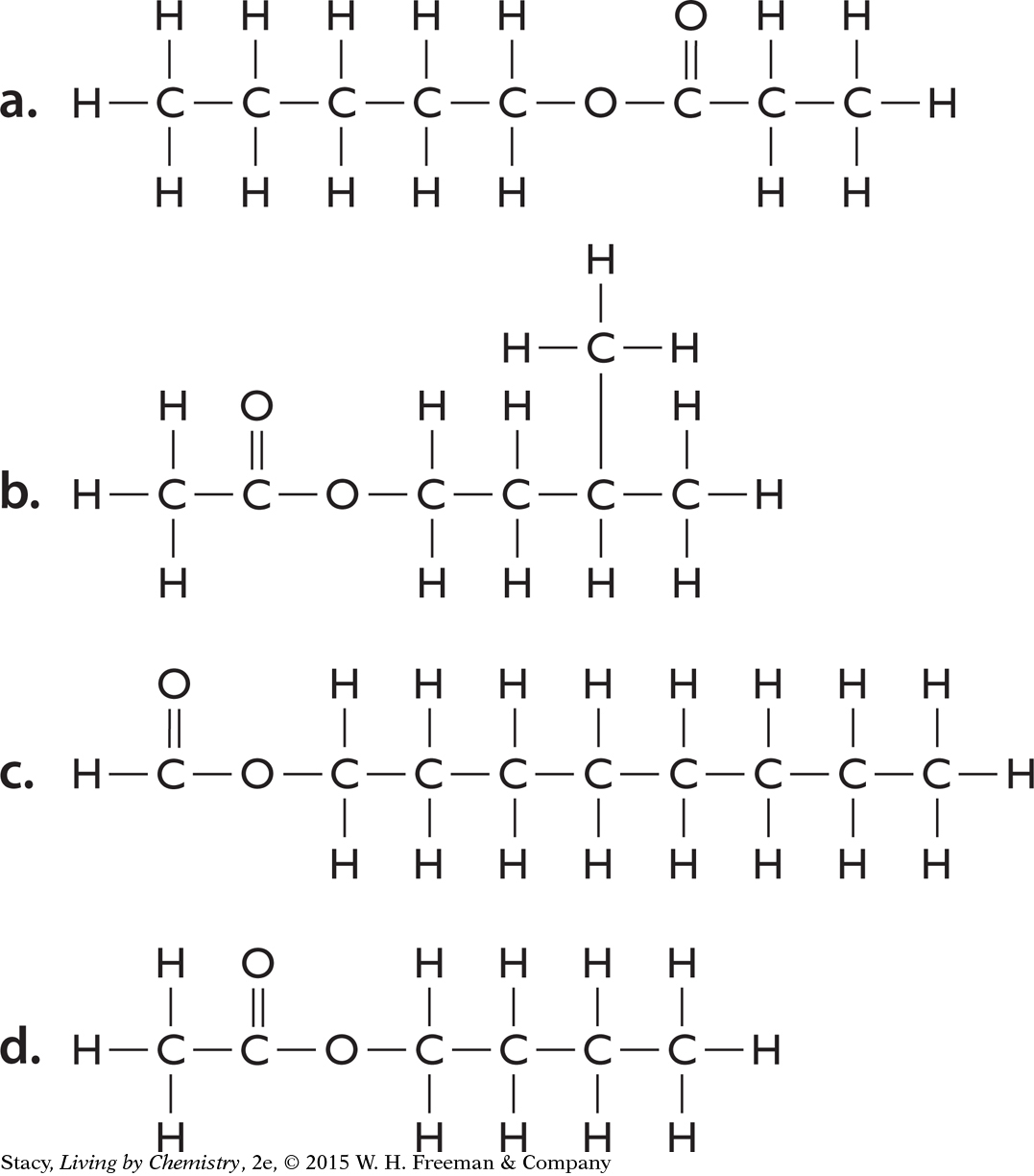
Ester molecules are named for the alcohol and acid that form them using the convention
(alcohol name)yl (acid name)ate
For example, methanol + ethanoic acid combine to make methyl ethanoate. Name the esters formed from
isopropanol + methanoic acid
caproic acid + butanol
salicylic acid + ethanol