LESSON 29: Molecules In Two Dimensions: Structural Formulas
151
THINK ABOUT IT

Alberto Pérez Veiga/Shutterstock
|

© RedHelga/iStockphoto
|
Would you rather smell sweaty gym socks or fresh pineapple? The choice is pretty clear. But what if you were asked if you would rather smell ethyl butyrate or hexanoic acid? How would you know which compound is safe to sniff? Both molecules have the same molecular formula: C6H12O2.
How can molecules with the same molecular formula be different?
To answer this question, you will explore
Predicting Smells
Structural Formula
Orientations of Structural Formulas
Predicting Smells
EXPLORING THE TOPIC
Predicting Smells
In Lesson 28: Sniffing Around, you arrived at some generalizations that relate smell to molecular formula and chemical name. What happens if you use these generalizations to make predictions about some new compounds? Consider the five new compounds listed in the table.
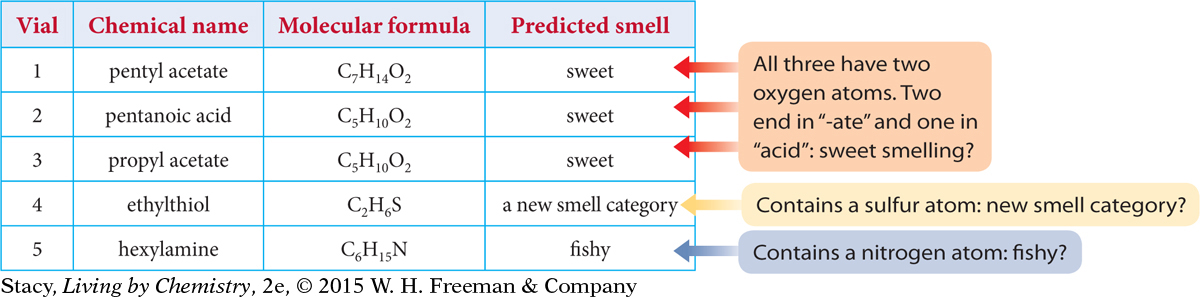
The predictions based on molecular formula and chemical name alone are reasonably accurate. Four out of five are correct. Ethylthiol has a skunk smell, so it belongs in a new smell category. The only prediction that is incorrect is the pentanoic acid in vial 2. It has a molecular formula identical to the molecular formula of a sweet-smelling molecule, but it has a very putrid smell. Apparently, two compounds can have identical formulas, different names, and very different smells. Perhaps this is why its name does not end in “-ate” like the other sweet-smelling compound.
152
As you continue to study molecules, some of the patterns discovered here will hold up, while others will be revised or abandoned. You can continue to refine your ideas about smell so that you can better understand the chemistry of smell.
Big Idea
Big Idea
Science involves coming up with ideas based on observations and then refining these ideas based on further observation.
Structural Formula
Structural Formula
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The spray of a skunk is a defensive adaptation. The spray contains the compound ethylthiol. Predators will usually leave a skunk alone once they have been blasted with this stinky substance that burns the eyes and nose and is difficult to get rid of.

As it turns out, two compounds can have identical molecular formulas but smell much different. This is because having the same molecular formula does not guarantee that the molecules themselves are identical.
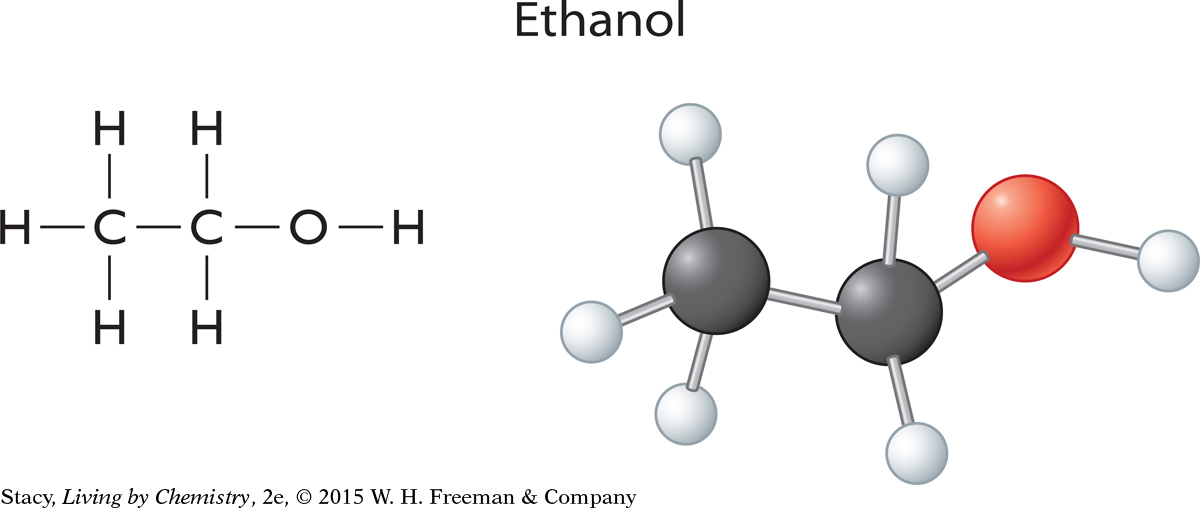
To show how the individual atoms in a molecule are bonded to each other, chemists use what is called a structural formula. A structural formula is a diagram or drawing that shows how the atoms in a molecule are arranged and where they are connected. The lines between the atoms represent covalent bonds holding the atoms together. Take a moment to examine the structural formulas for ethyl butyrate and hexanoic acid below. What differences do you see?
These compounds have the same molecular formula, but different smells.
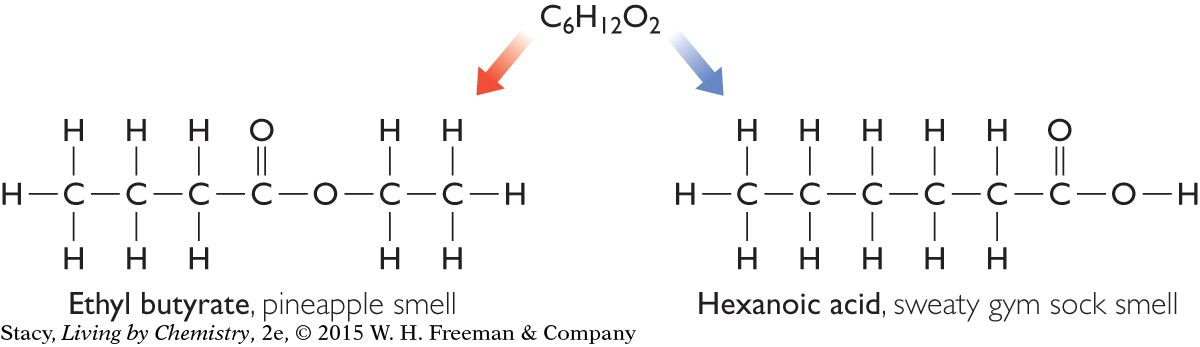
Notice that the oxygen atoms are located in different places within the two molecules. In one molecule, the oxygen atoms are in the middle of the molecule, whereas in the other molecule, the oxygen atoms are at one end.
ISOMERS
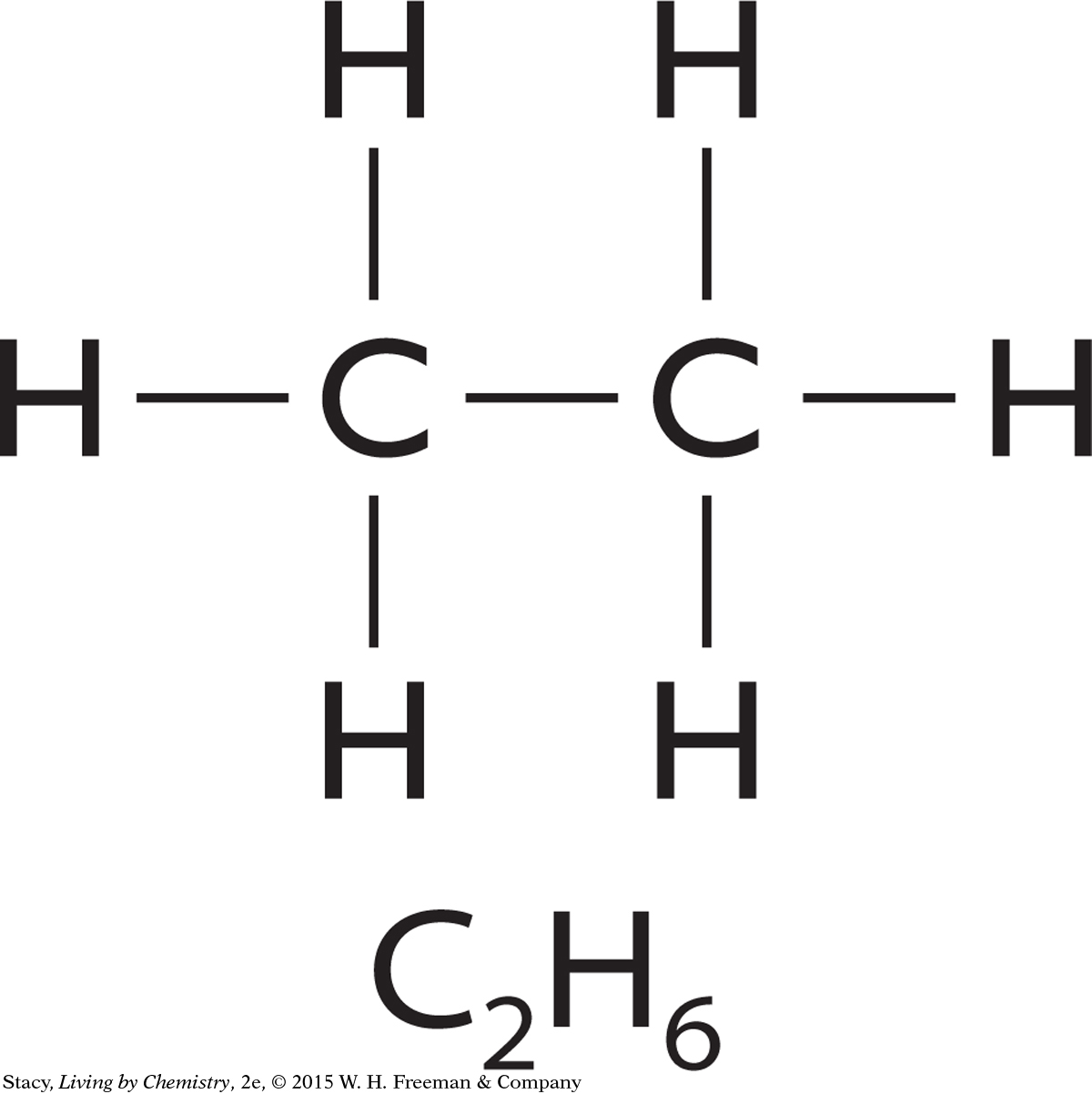
In some compounds, the atoms can be connected only a certain way. For instance, the formula C2H6 has only one structural formula.
As the number and types of atoms in a molecule increase, there are more possible ways to connect the atoms. Molecules with the same molecular formula and different structural formulas are called isomers. The formula C2H6O has two isomers, shown here.
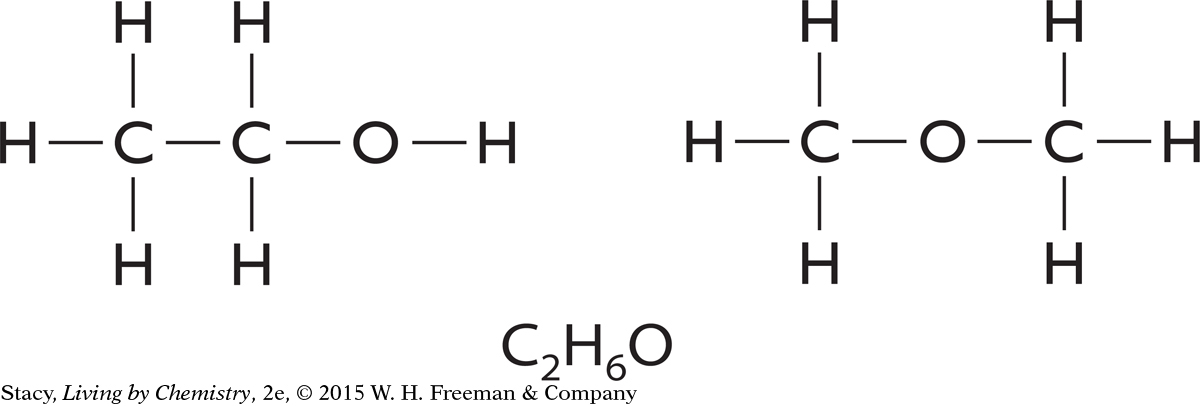
153
Ethyl butyrate and hexanoic acid are considered isomers of each other. The formula C6H12O2 has many more isomers. In fact, there are more than 25 different molecules with the molecular formula C6H12O2, each with unique properties.
Important to Know
Structural formulas are used to represent molecules that are covalently bonded. They are not used to represent ionic compounds.
Drawings of structural formulas are flat and two-dimensional. However, molecules are not flat. They take up three dimensions in space.
Orientations of Structural Formulas
Orientations of Structural Formulas
There are several ways to draw the same structural formula. Consider these two drawings.
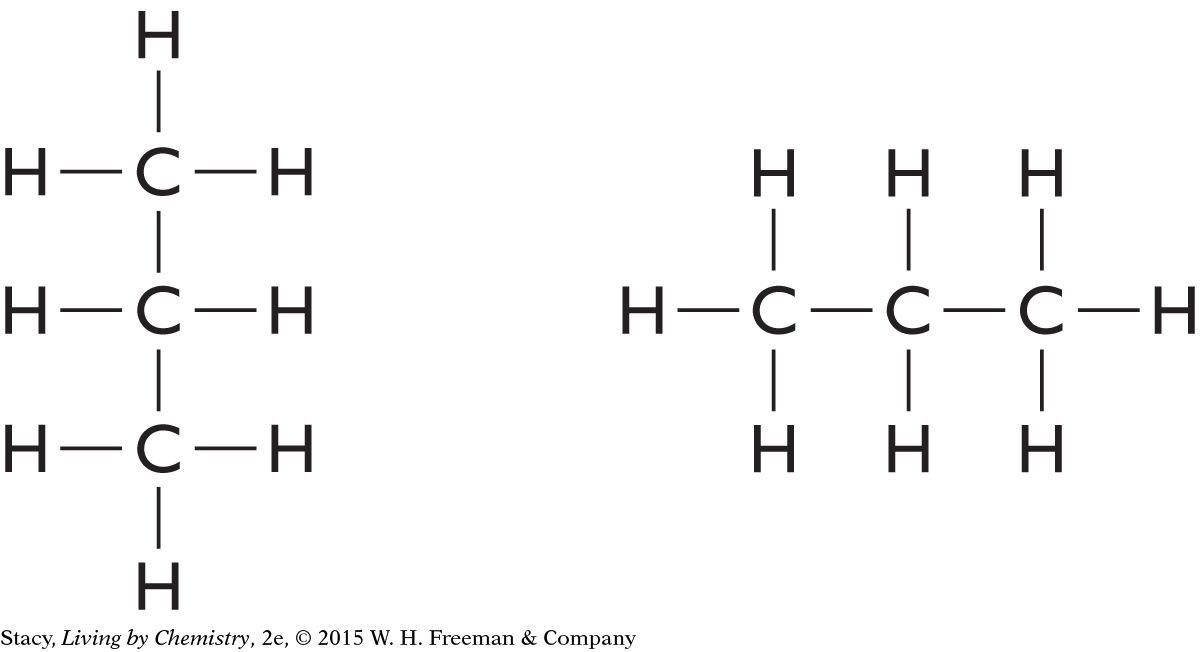
These two molecules have the same molecular formula and each atom has the same connections. The structural formula has just been turned 90º in space. These molecules are not isomers; they have the same structural formula.
Here are four structural formulas. How many different compounds are represented?
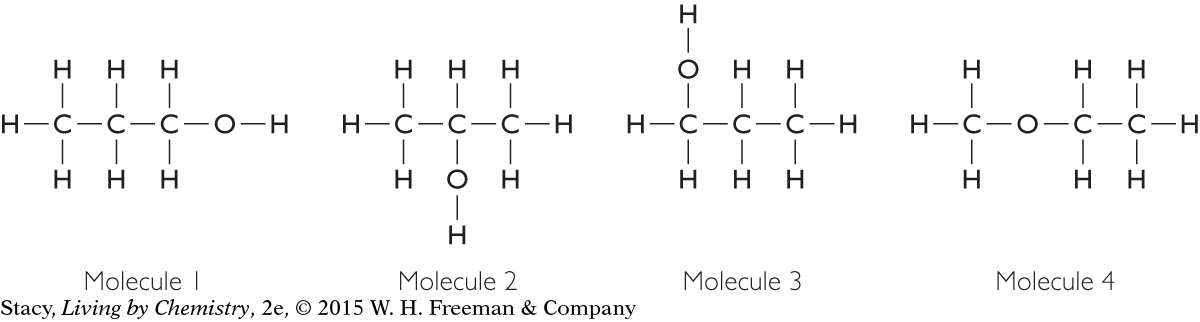
If you build three-dimensional models of these molecules, you will find that there are only three different compounds here. The first and the third structural formulas are identical. All the atoms in the first and third structures are connected in the same way.
The illustration on the next page shows how molecule 1 can be turned and rotated to match molecule 3 without taking the model apart.
154
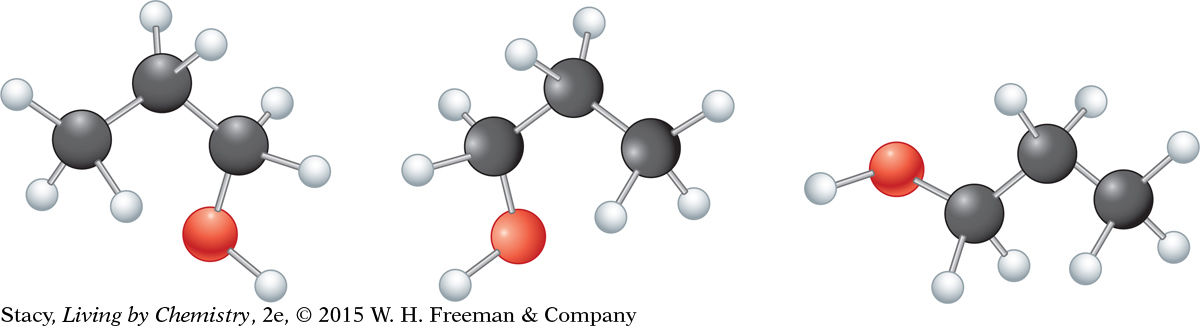
Structural formulas show only the sequence in which one atom is connected to the next. The way atoms are connected in a molecule is like a charm bracelet. The middle charm is always between the same two charms regardless of how you hold the bracelet. It does not matter if the oxygen atom is drawn on the left or right side of the molecule. It also does not matter if the O—H is drawn horizontally or vertically. It is only the number and type of connections that matter.
Example
Isomers of C3H9N
How many different isomers are represented below?
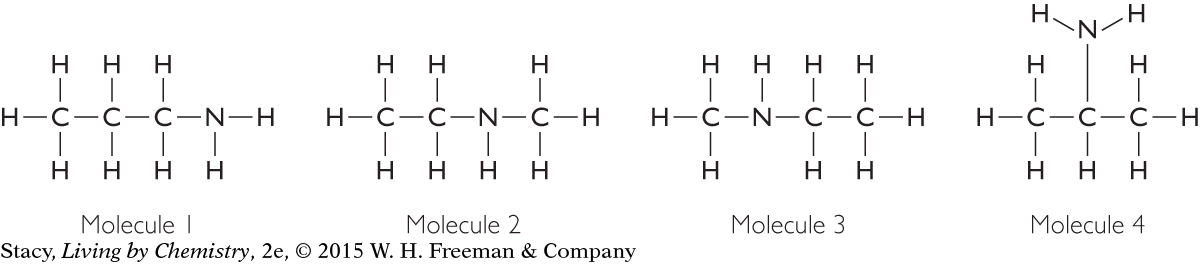
Solution
There are three isomers. Molecules 2 and 3 are identical. So there are three different compounds represented here.
LESSON SUMMARY
LESSON SUMMARY
How can molecules with the same molecular formula be different?
KEY TERMS
structural formula
isomer
Compounds with identical molecular formulas may have different structures. Chemists use drawings called structural formulas to show how the atoms in molecules are connected to each other. Each line in a structural formula represents a covalent bond between atoms. When molecules have the same molecular formula but different structural formulas, they are called isomers. Isomers represent different compounds with different properties. Because smell is a property, compounds with the same molecular formula may smell different from each other because they are different substances.
155
Exercises
Reading Questions
What information does a structural formula provide?
What are isomers?
Reason and Apply
If you are given a structural formula of a molecule, do you also know its molecular formula? Explain your thinking.
The words isotope and isosceles also have the prefix “iso-.” How are their meanings similar to that of the word isomer?
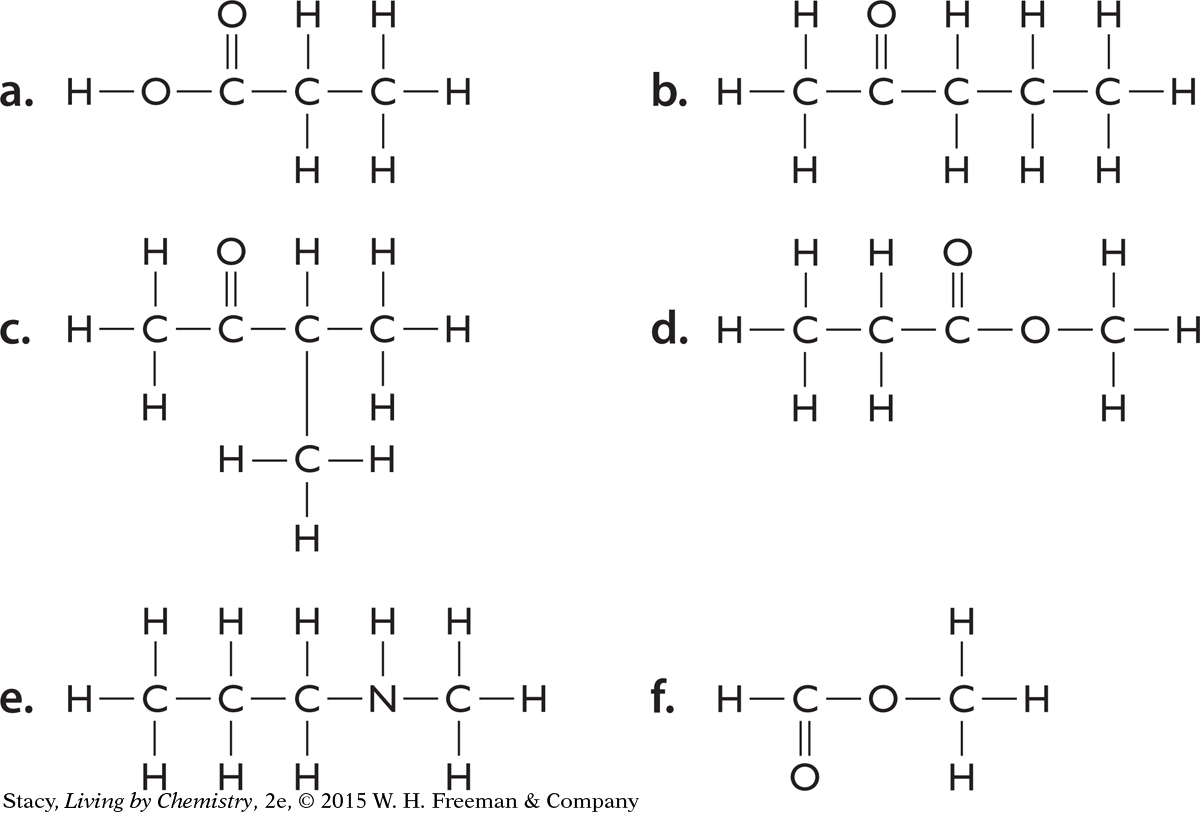
Structural formulas for six molecules are shown below. Write the molecular formula for each of the molecules.
Two molecules have the same molecular formula, yet one smells sweet and the other smells putrid. Explain how you think this might be possible.
For each of the molecules in Exercise 5, draw an isomer of the compound.