LESSON 30: HONC If You Like Molecules: Bonding Tendencies
THINK ABOUT IT
To understand the chemistry of smell, it is necessary to understand how molecules are put together. If you examine the structural formulas of molecules, you can see patterns in the way the atoms are connected. For example, hydrogen atoms are always arranged around the outside of the molecule. Plus, hydrogen atoms always have only one line connecting them to other atoms, while carbon atoms always have more than one line connecting them to other atoms.
What are the rules for drawing structural formulas?
To answer this question, you will explore
The HONC 1234 Rule
Drawing Structural Formulas
The HONC 1234 Rule
EXPLORING THE TOPIC
The HONC 1234 Rule
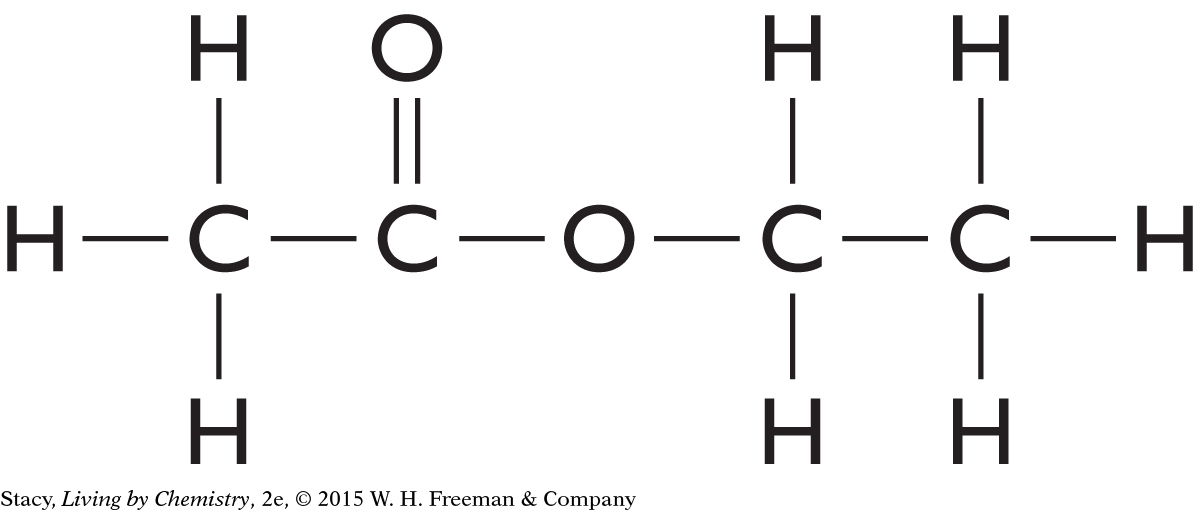
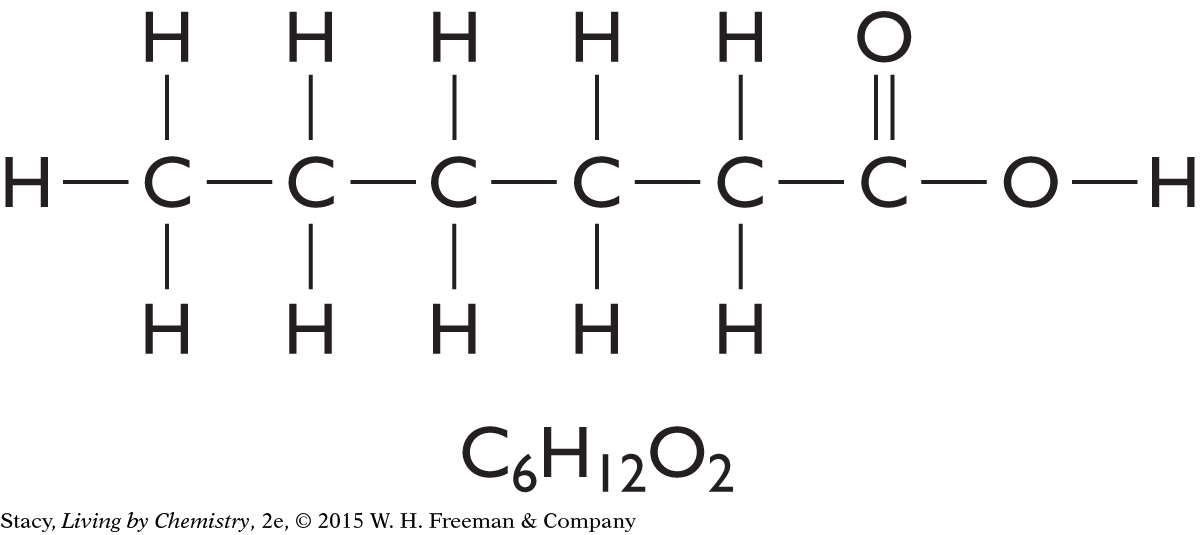
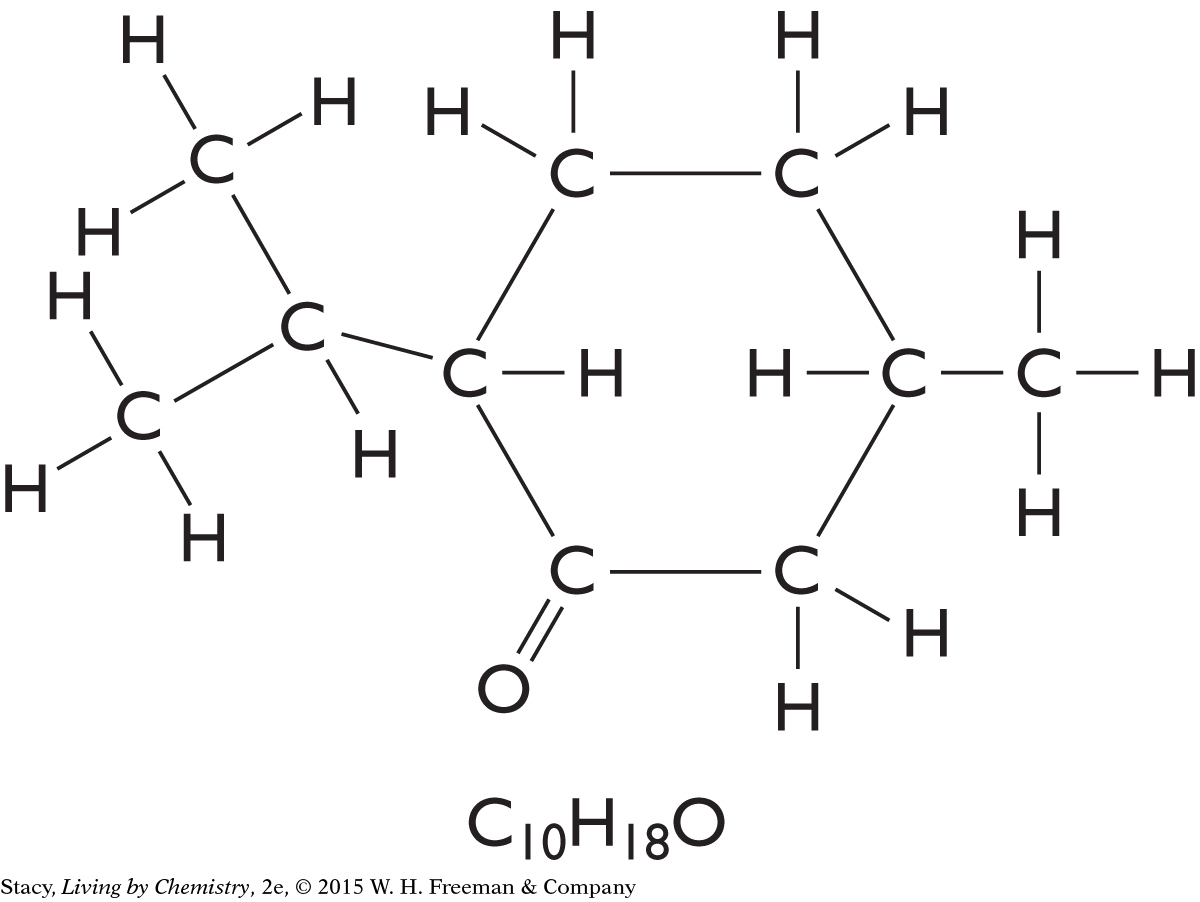
The structural formulas for three molecules are shown here. Take a moment to count the number of times each type of atom—hydrogen, oxygen, nitrogen, and carbon—is connected to other atoms.
|
|
|
Every Hydrogen atom has one line connecting it to other atoms.
Every Oxygen atom has two lines connecting it to other atoms.
Every Nitrogen atom has three lines connecting it to other atoms.
Every Carbon atom has four lines connecting it to other atoms.
This information is sometimes referred to as the HONC 1234 rule. Within most molecules, hydrogen makes one bond, oxygen makes two bonds, nitrogen makes three bonds, and carbon makes four bonds. The bonds in a structural formula are represented by lines. One line connecting two atoms is called a single bond. A pair of lines connecting the same two atoms, as in C==O, is called a double bond.
The HONC 1234 rule tells you how four of the most common nonmetal atoms will bond. You will learn about the bonding of other nonmetal atoms like sulfur, S, and chlorine, Cl, in later lessons.
Example 1
HONC 1234
Are the following molecules correct according to the HONC 1234 rule? If not, what is wrong with them?
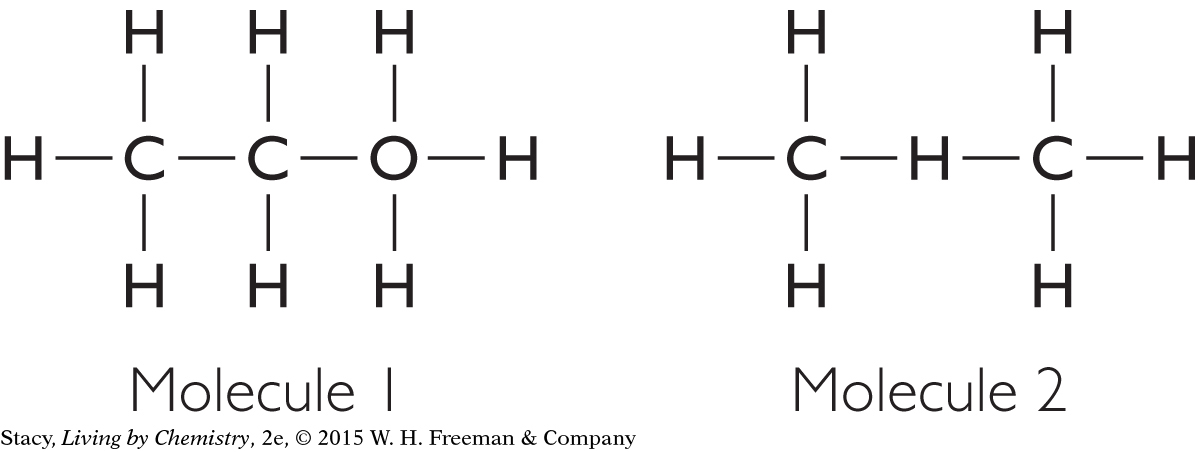
Solution
Both molecules are incorrect according to the HONC 1234 rule. In molecule 1, there is an oxygen atom with four bonds. It should have only two bonds. In molecule 2, there is a hydrogen atom with two bonds. It should have only one. The hydrogen atom cannot go in the middle of the molecule, between two carbon atoms.
Drawing Structural Formulas
Drawing Structural Formulas
The HONC 1234 rule is all you need to draw the structural formulas for thousands of different molecules correctly. Some examples are given here and on top of the next page.
STRUCTURAL FORMULA FOR C2H6
ASTRONOMY CONNECTION
ASTRONOMY
CONNECTION
At room temperature, ethane, C2H6, is a flammable gas. On Earth, ethane is one of the components of natural gas. It has also been detected in the atmospheres of Jupiter, Saturn, Uranus, and Neptune.
Consider a molecule with two carbon atoms and six hydrogen atoms. Its molecular formula is C2H6. What structural formula would this molecule have? Start by connecting the two carbon atoms with a single bond. The “carbon backbone” is generally a good place to start when drawing molecules.
| Step 1: Connect the carbon atoms: | C—C |
| Step 2: Add hydrogen atoms. Make sure each hydrogen atom has just one bond and each carbon atom has a total of four bonds: |
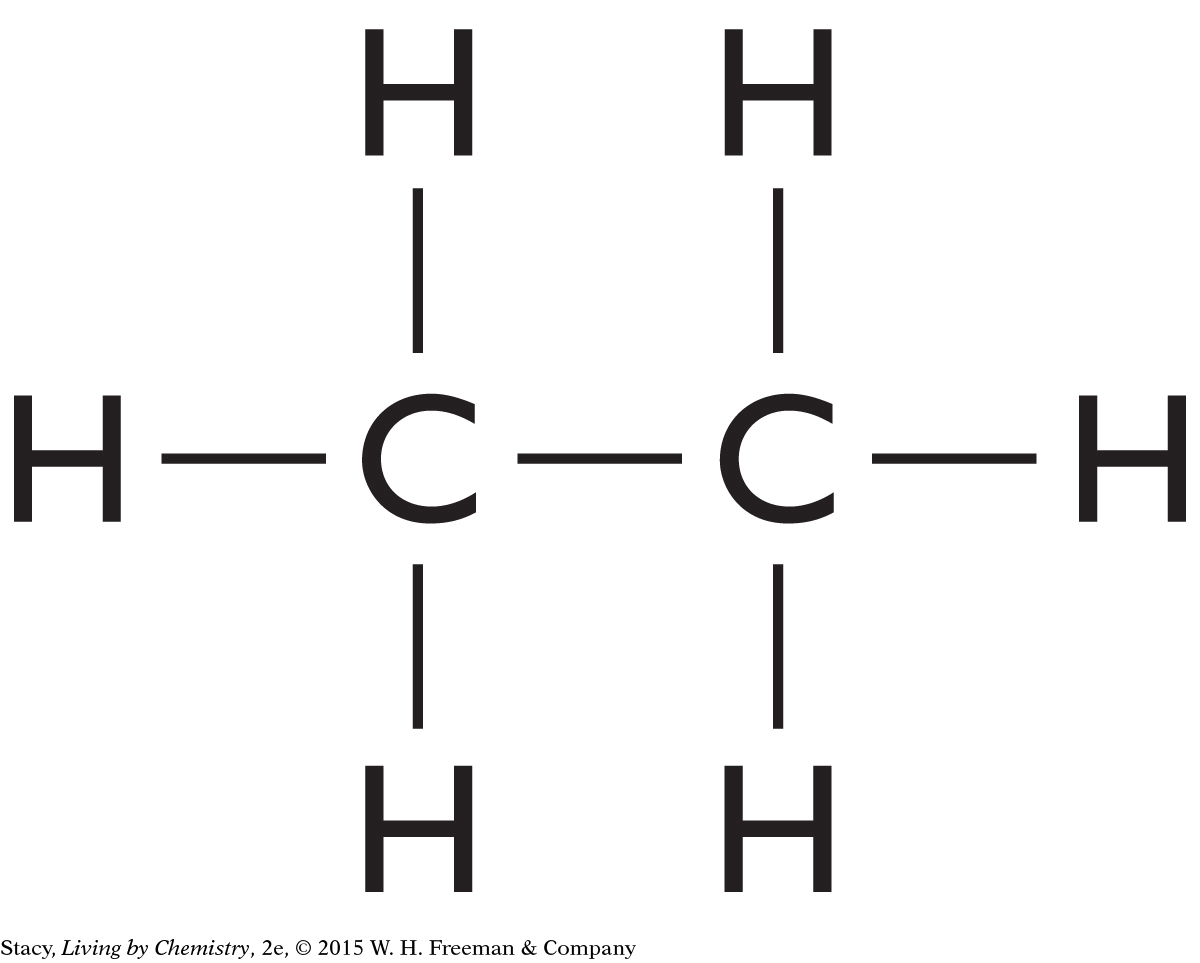
|
The structural formula shown above is consistent with the HONC 1234 rule. This is the only structure you can make out of this molecular formula. It represents a substance called ethane.
STRUCTURAL FORMULA FOR C2H6O
Consider a molecule with two carbon atoms, six hydrogen atoms, and one oxygen atom. Its molecular formula is C2H6O. What structural formula could this molecule have?
Start by connecting the carbon atoms. Then add the oxygen atom.
| Step 1: Connect the carbon atoms: | C—C |
| Step 2: Add the oxygen atom to the carbon chain: | C—C—O |
| Step 3: Add hydrogen atoms last: |
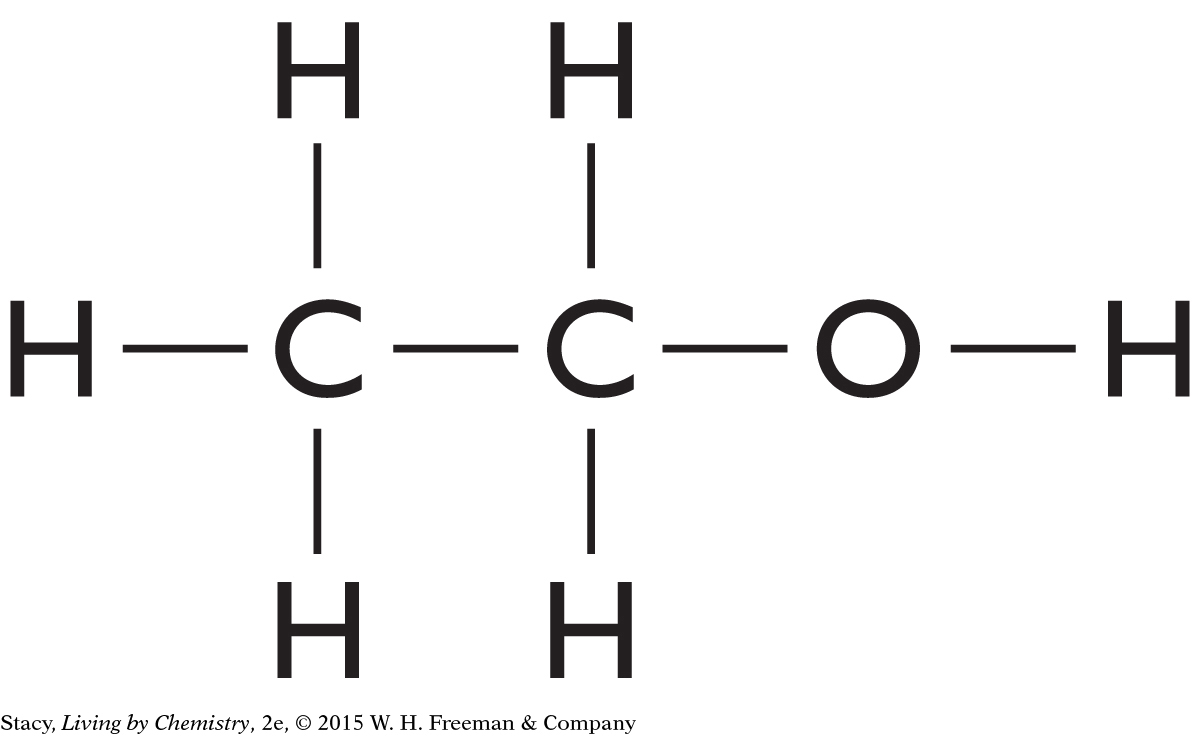
|
Make sure your structural formula follows the HONC 1234 rule. Notice that hydrogen atoms are generally the last atoms you add when creating a structural formula.
CONSUMER CONNECTION
CONSUMER
CONNECTION
Ethanol, C2H6O, can be made from plants such as sugarcane, corn, and switchgrass, and can be used as an alternative fuel for automobiles. It can be used alone or blended with gasoline. However, it currently takes about a liter of fuel to produce one liter of ethanol, which may offset its environmental benefit.

This molecule is called ethanol or ethyl alcohol.
A SECOND STRUCTURE—AN ISOMER
Ethanol is not the only possible structure for this particular molecular formula. The atoms in C2H6O can also be arranged a different way, to form a completely different molecule. This new structure is created by placing the oxygen atom between the two carbon atoms.
| Step 1: Connect the carbon and oxygen atoms: | C—O—C |
| Step 2: Add hydrogen atoms: |
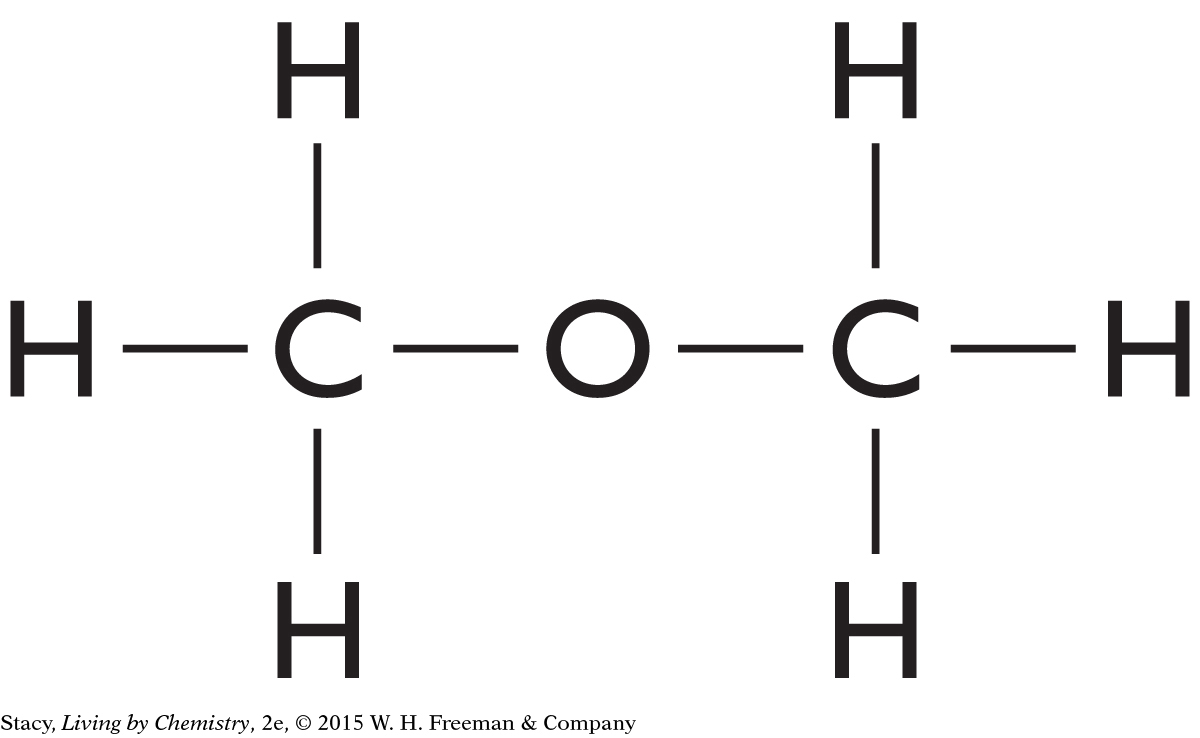
|
This structure also follows the HONC 1234 rule and is a correct structural formula for C2H6O. This molecule is called dimethyl ether. Ethanol and dimethyl ether are isomers. They have the same molecular formula but different structural formulas. Dimethyl ether has different properties from ethanol, including smell.
Not all structural formulas are simple to figure out. As the number and type of atoms increase, there are more possible ways to connect the atoms.
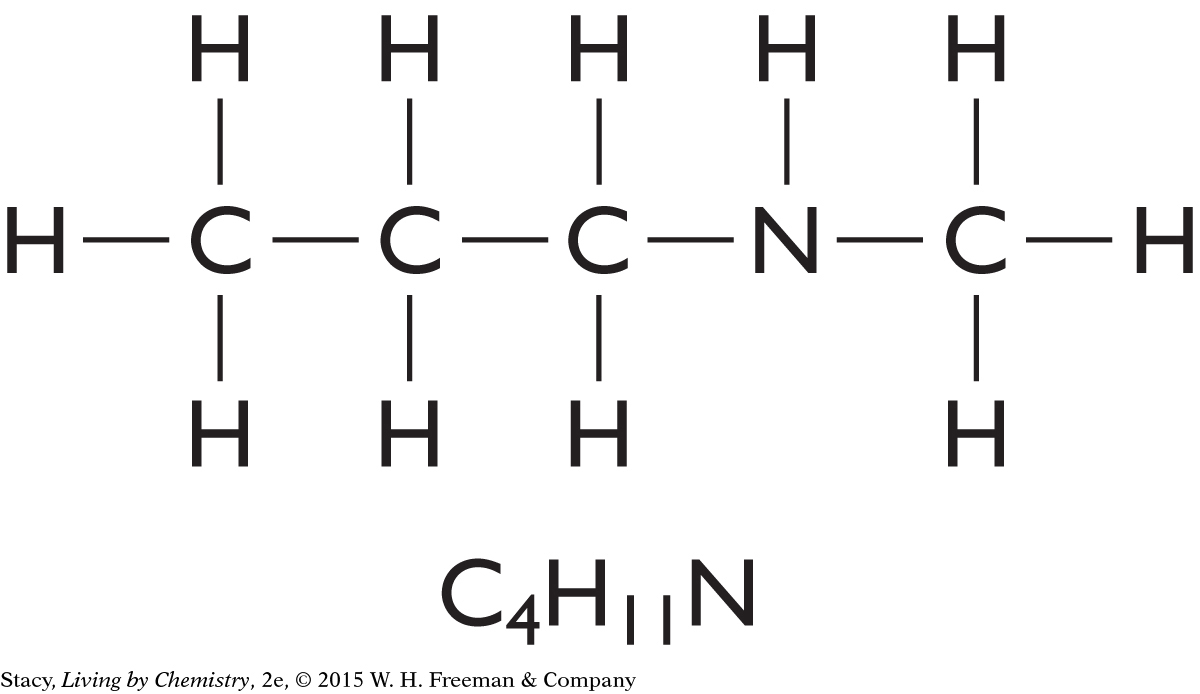
Example 2
Structural Formula for C4H11N
Draw a structural formula for the molecular formula C4H11N.
Solution
Start by making a chain of the carbon atoms. Place the nitrogen atom anywhere in the chain. Finish by adding hydrogen atoms so that every carbon atom has four bonds and the nitrogen atom has three bonds. Two possible solutions are shown here, but many more are possible.
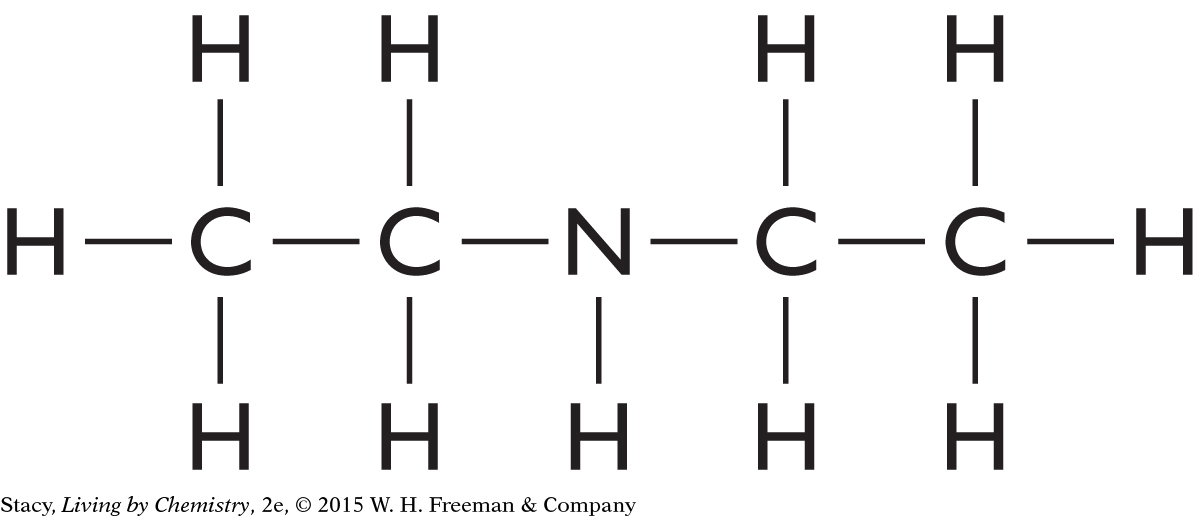
|
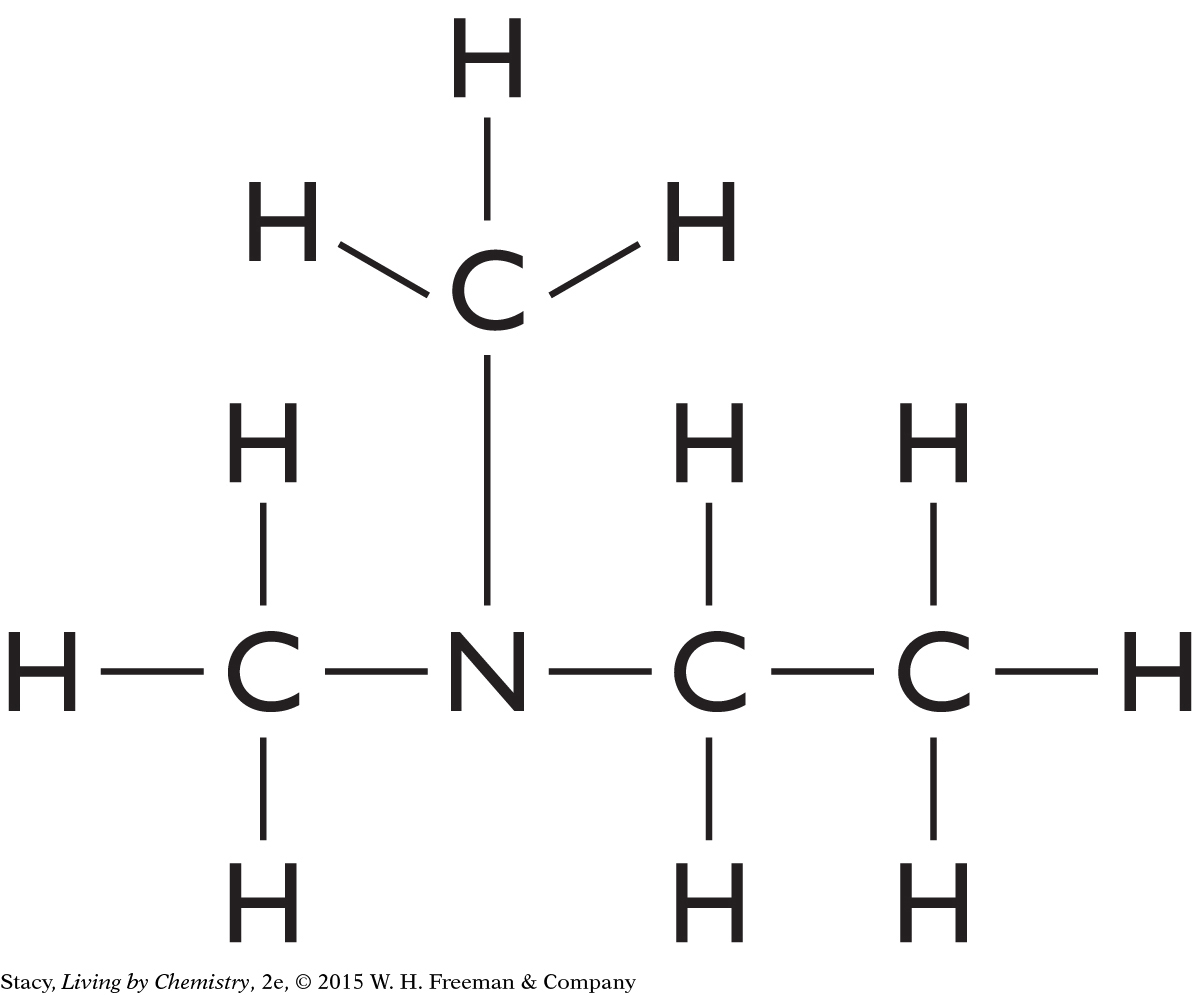
|
LESSON SUMMARY
LESSON SUMMARY
What are the rules for drawing structural formulas?
KEY TERM
HONC 1234 rule
The individual atoms in molecules are not connected randomly. Each atom in a molecule has a tendency to bond a specific number of times. The HONC 1234 rule describes the bonding patterns of hydrogen, oxygen, nitrogen, and carbon atoms. The HONC 1234 rule can be a valuable tool for creating structural formulas from molecular formulas.
Exercises
Reading Questions
What is the HONC 1234 rule?
Explain why one molecular formula can represent more than one structural formula.
Reason and Apply
Use the HONC 1234 rule to create possible structural formulas for molecules with these molecular formulas. Remember that it is easiest to start with the carbon atoms.
C3H8O2
C4H11N
C4H10
C5H12O
Is each structural formula correct according to the HONC 1234 rule? For any molecules that don’t follow the HONC 1234 rule, repair the incorrect structural formula. (Note: There may be more than one correct way to repair a formula.)
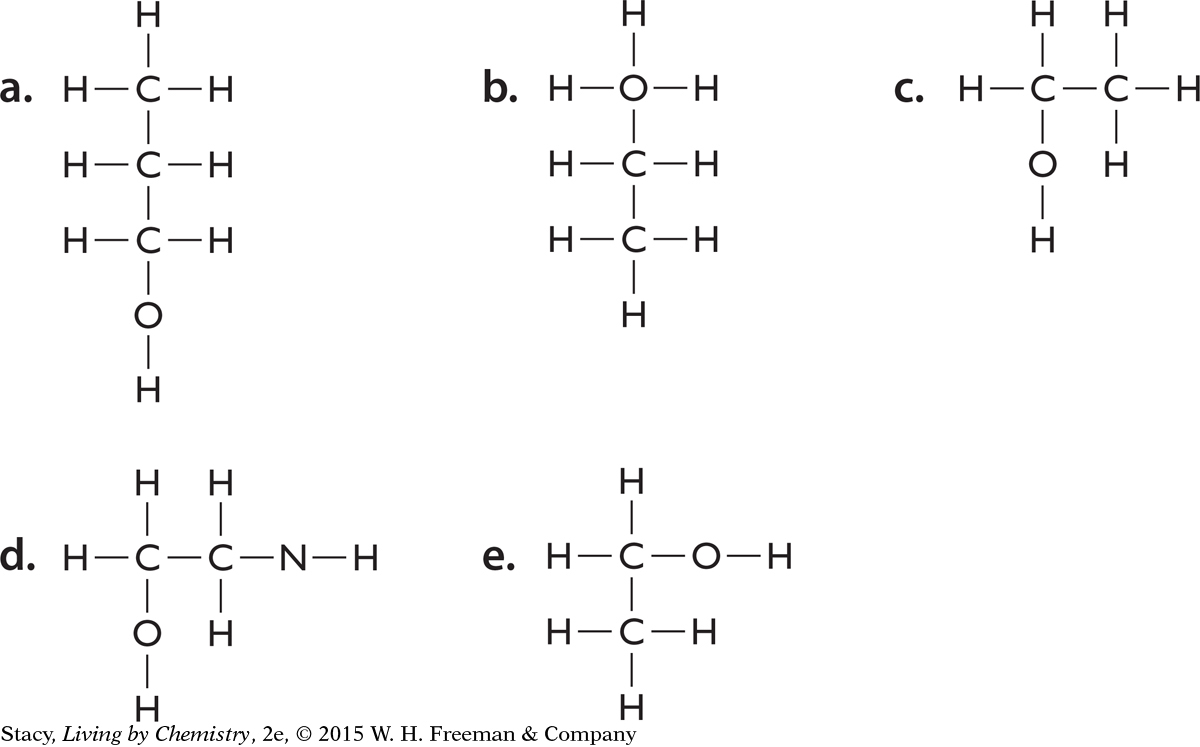
Think about how molecules might interact with your nose. Why do you think molecules with different structural formulas have different smells?