LESSON 32: Eight Is Enough: Octet Rule
165
THINK ABOUT IT
When atoms bond covalently to form molecules, they share electrons to obtain an electron arrangement similar to that of a noble gas atom. Lewis dot structures can help you to discover how atoms share electrons to form molecules.
How do atoms bond to form molecules?
To answer this question, you will explore
The Octet Rule
Double and Triple Bonds
The Octet Rule
EXPLORING THE TOPIC
The Octet Rule
When carbon, nitrogen, oxygen, and fluorine combine with hydrogen, they form these molecules:
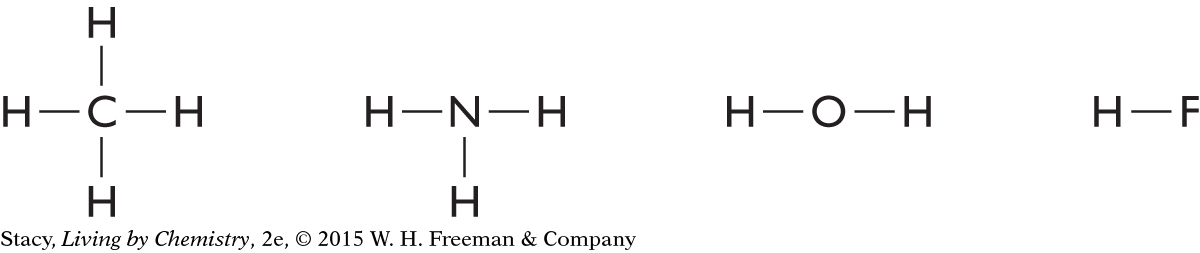
All of the above compounds are extraordinarily different. However, their Lewis dot structures reveal a striking similarity. Once carbon, nitrogen, oxygen, and fluorine are bonded, they are each surrounded by eight valence electrons.
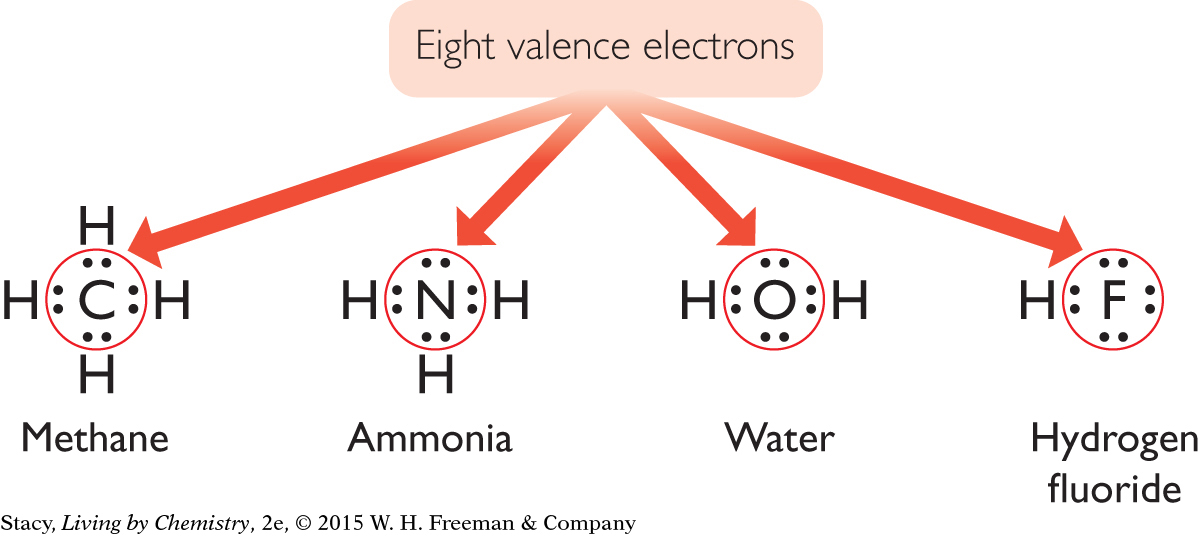
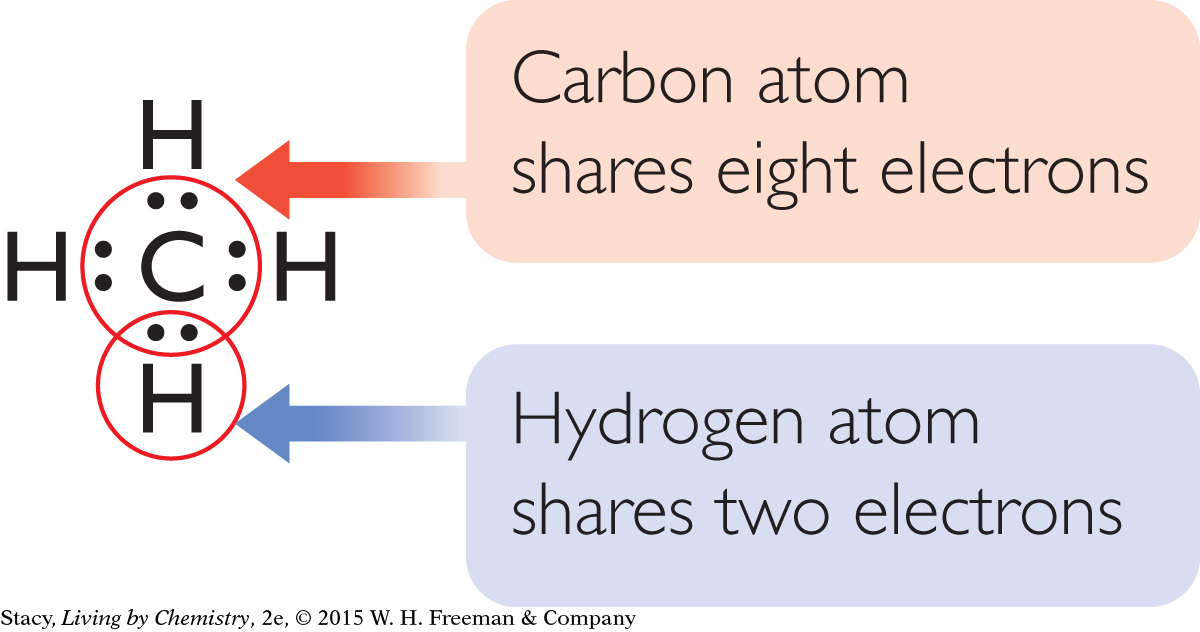
This tendency to bond until eight valence electrons surround an atom is called the octet rule. (The word octet comes from octo, which is Latin for “eight.”) After these atoms are bonded, they all resemble atoms of a noble gas in their electron arrangements. Recall that the electron arrangements of the noble gases are very stable.
166
CONSUMER CONNECTION
CONSUMER
CONNECTION
Saturated fats are “saturated” because the carbon atoms are bonded with as many hydrogen atoms as possible. Saturated fats are found in meat, dairy products, and tropical oils such as palm oil and coconut oil, and are usually solid at room temperature. In contrast, vegetable oils are usually monounsaturated or polyunsaturated, meaning that they have one or more double bonds between carbon atoms and are bonded with fewer hydrogen atoms. They are usually liquid at room temperature.

Note that hydrogen is an exception to the octet rule. Each hydrogen atom shares two electrons, not eight. A covalently bonded hydrogen atom resembles the noble gas helium, He, which has only two valence electrons.

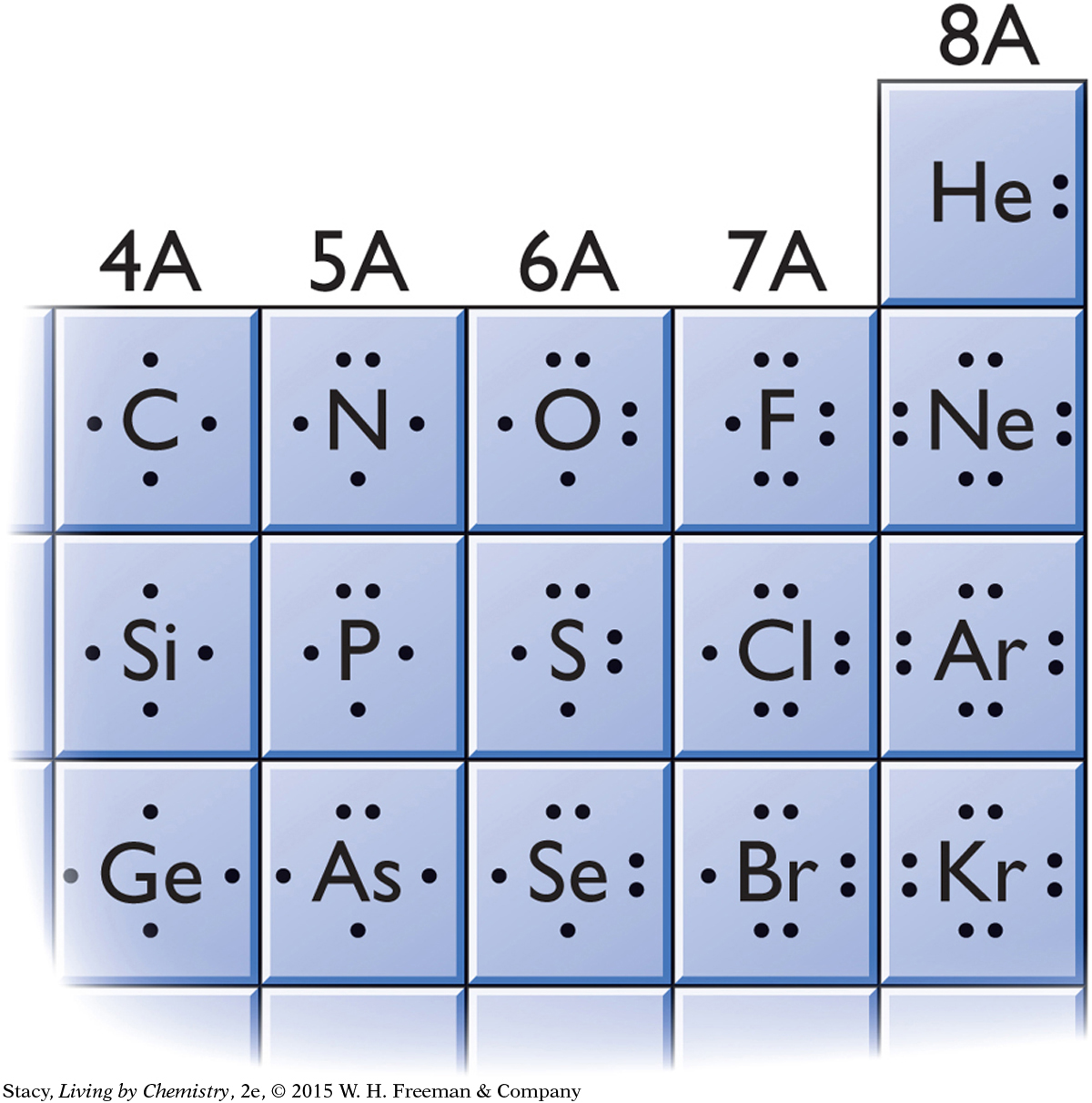
PERIODIC TRENDS
To predict covalent bonding in molecules, you can use the periodic table. In the illustration, Lewis dot symbols are shown for nonmetal elements in the second, third, and fourth rows. Notice that the elements in the same group have similar Lewis dot symbols and bond in a similar way. For example, selenium, Se, which is below sulfur, S, and oxygen, O, has two unpaired electrons and forms two bonds, just like oxygen.
Double and Triple Bonds
Double and Triple Bonds
There is more than one way to satisfy the octet rule through bonding. Quite a few of the structural formulas you have examined so far have a bond with two lines. This type of bond is called a double bond. In a Lewis dot structure, a double bond is represented by four dots instead of the usual two. A double bond contains four electrons that are shared between atoms. Methyl methanoate, C2H4O2, is an example of a molecule that contains a double bond. Its structural formula and Lewis dot structure are shown here.
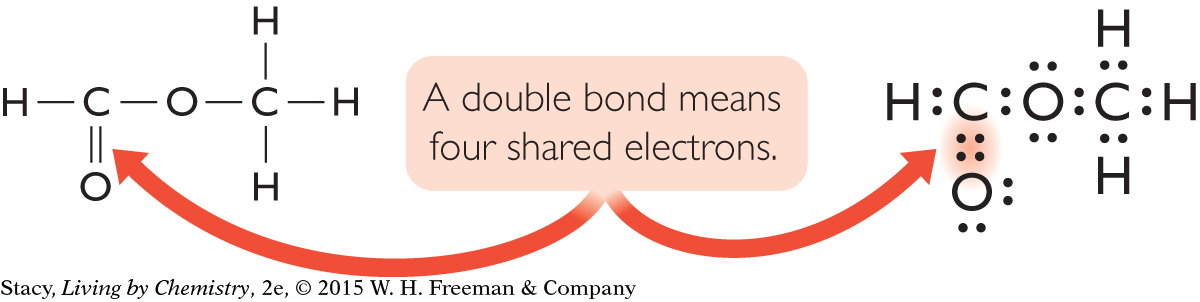
The carbon and oxygen atoms with double bonds are surrounded by a total of eight valence electrons each, just like the atoms with single bonds.
167
Example
Carbon Dioxide
Draw the Lewis dot structure and structural formula for carbon dioxide, CO2.
Solution
Step 1: Start with the Lewis dot symbols. Bring the atoms together.

Step 2: Check to see if the octet rule is satisfied. This Lewis dot structure isn’t correct. Move the remaining unpaired electrons to create double bonds.
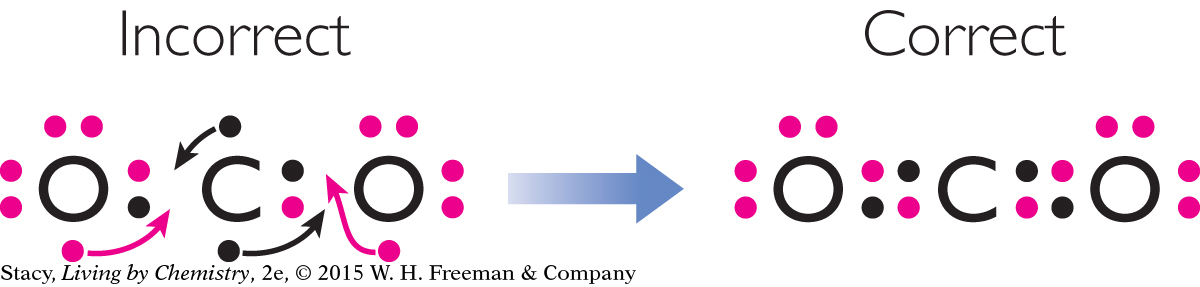
Step 3: Check your molecule again to see that each atom in it satisfies the octet rule and still has the correct number of valence electrons.
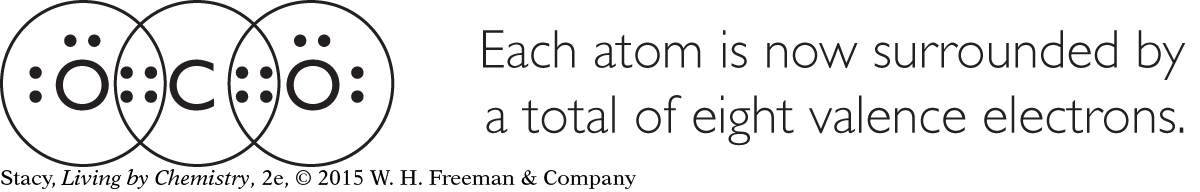
Step 4: To make the structural formula, replace each pair of dots with a line.
O=C=O
If two atoms can share four electrons, can they share six electrons, or even more? The answer is yes.
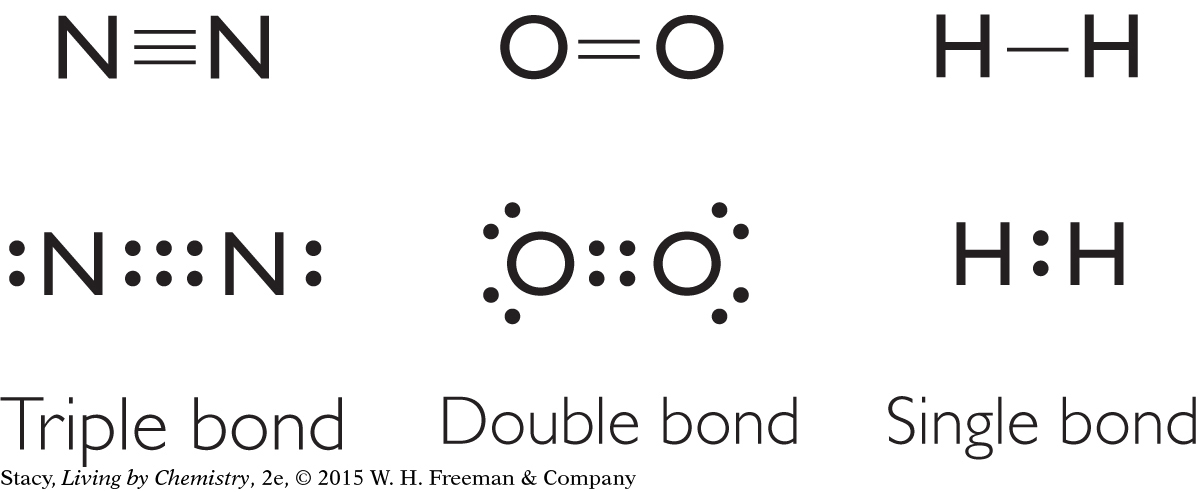
Nitrogen gas, N2, has a triple bond between the two nitrogen atoms, so each nitrogen atom has six shared valence electrons. Notice that there are also two lone pairs in this molecule. Oxygen gas, O2, has a double bond between the oxygen atoms. The four lone pairs in the oxygen molecule have been adjusted slightly in this Lewis dot structure to space the electrons more evenly around the atoms. The placement of the dots in a Lewis dot structure can vary as long as the number of pairs of electrons is correct. Quadruple bonds are also possible, but quite rare.
Some of the most common substances in the world around you are molecules with double and triple bonds. The air you breathe is composed mainly of nitrogen gas and oxygen gas. In fact, the air is 78% nitrogen gas.
LESSON SUMMARY
LESSON SUMMARY
How do atoms bond to form molecules?
KEY TERMS
octet rule
double bond
triple bond
When nonmetal atoms bond, they share electrons to obtain the same electron arrangement as a noble gas atom. Nonmetal atoms will share electrons with other atoms so that both atoms share a total of eight valence electrons each. This is called the octet rule. Hydrogen still fits the sharing pattern, but it ends up with only two valence electrons like the noble gas helium. Atoms can also satisfy the octet rule by forming double and triple bonds in which they share four or six valence electrons.
168
Exercises
Reading Questions
Explain why nitrogen bonds with hydrogen to form NH3, but not NH2 or NH4. Use Lewis dot structures to support your argument.
What is the octet rule, and how can you use it to create a molecular structure?
Reason and Apply
List three nonmetal elements that combine with only one fluorine atom to satisfy the octet rule.
List two nonmetal elements that combine with three hydrogen atoms to satisfy the octet rule.
Draw Lewis dot structures for these molecules. Notice that in part d and part f, the formulas are written in a way that emphasizes the structure of the molecule.
CF4
CH3Cl
SiCl2H2
CH3OH
HOCl
CH3NH2
Use the octet rule to draw Lewis dot structures for all the stable molecules with the molecular formula C3H8O. (Hint: There are three total molecules.)
Consider the molecules C2H2, N2H2, and H2O2.
Draw a Lewis dot structure for each one. What pattern do you notice?
What can you do to check that your Lewis dot structures are correct? Name at least two ways.
Consider the molecules C2H4 and N2H4. Draw a Lewis dot structure for each of the molecules.
Which is more likely to exist in nature, a molecule of CH3 or a molecule of CH4? Explain your reasoning.