LESSON 33: Where’s The Fun?: Functional Groups
169
THINK ABOUT IT
It makes sense that the structure of a molecule would affect its properties. The atoms in ethyl butyrate (pineapple smell) and hexanoic acid (dirty sock smell) are connected differently, so they behave differently when they enter your nose. But what about molecules that smell similar? What is it about these structures that causes them to have similar properties?
What does the structure of a molecule have to do with smell?
To answer this question, you will explore
Relating Smell to Molecular Structure
Functional Groups
Classifying Molecules
Relating Smell to Molecular Structure
EXPLORING THE TOPIC
Relating Smell to Molecular Structure
PUTRID-SMELLING MOLECULES
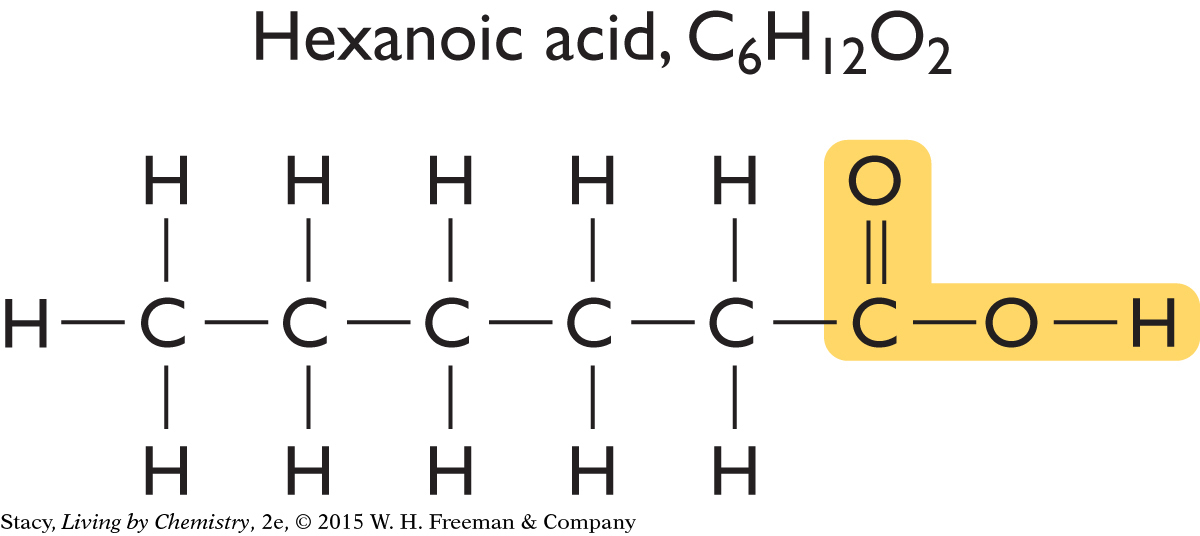
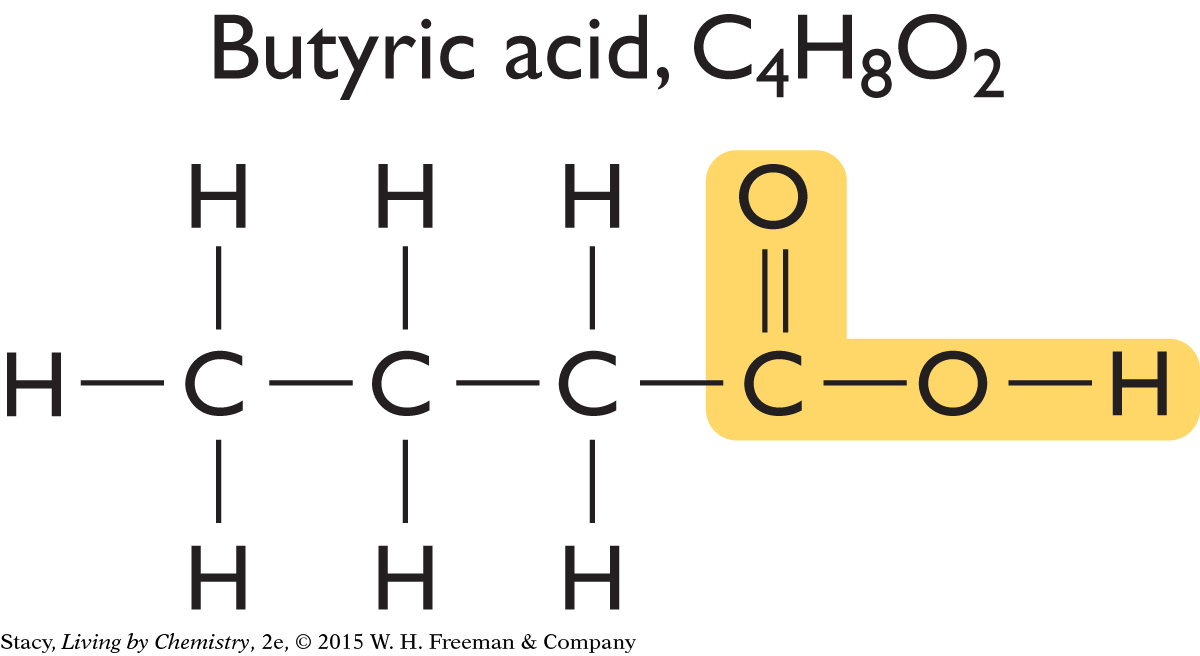
Consider two molecules in the same smell category. Both hexanoic acid and butyric acid smell putrid. The first smells like stinky feet while the second smells like a carton of spoiled milk. Take a moment to compare the structural formulas of hexanoic acid and butyric acid.
|
|
Notice that both molecules have two oxygen atoms bonded on the end in an identical fashion. They both contain the structural feature highlighted.
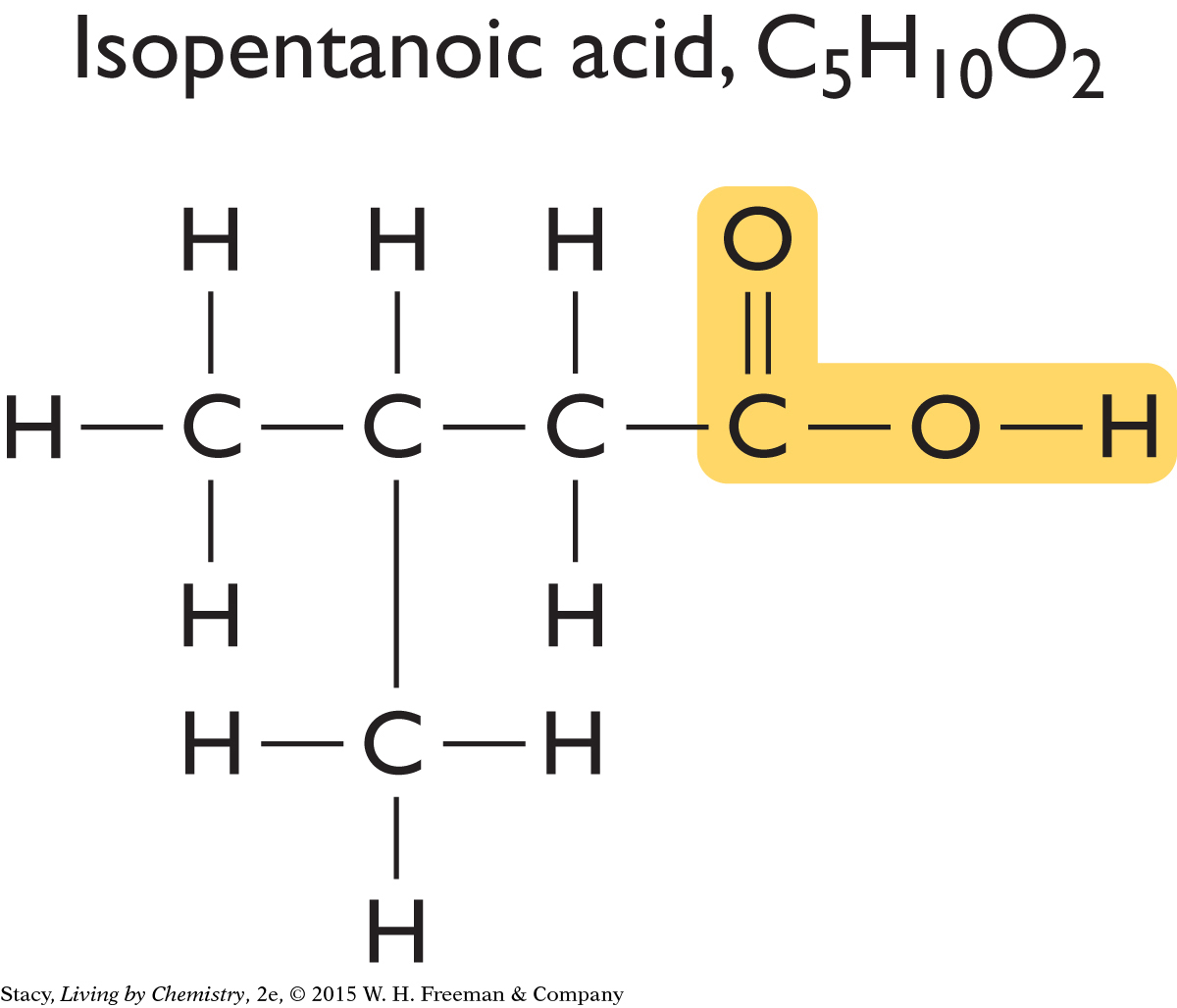
Perhaps all putrid-smelling molecules have this same structural feature. Two more compounds that smell putrid are shown here.
|
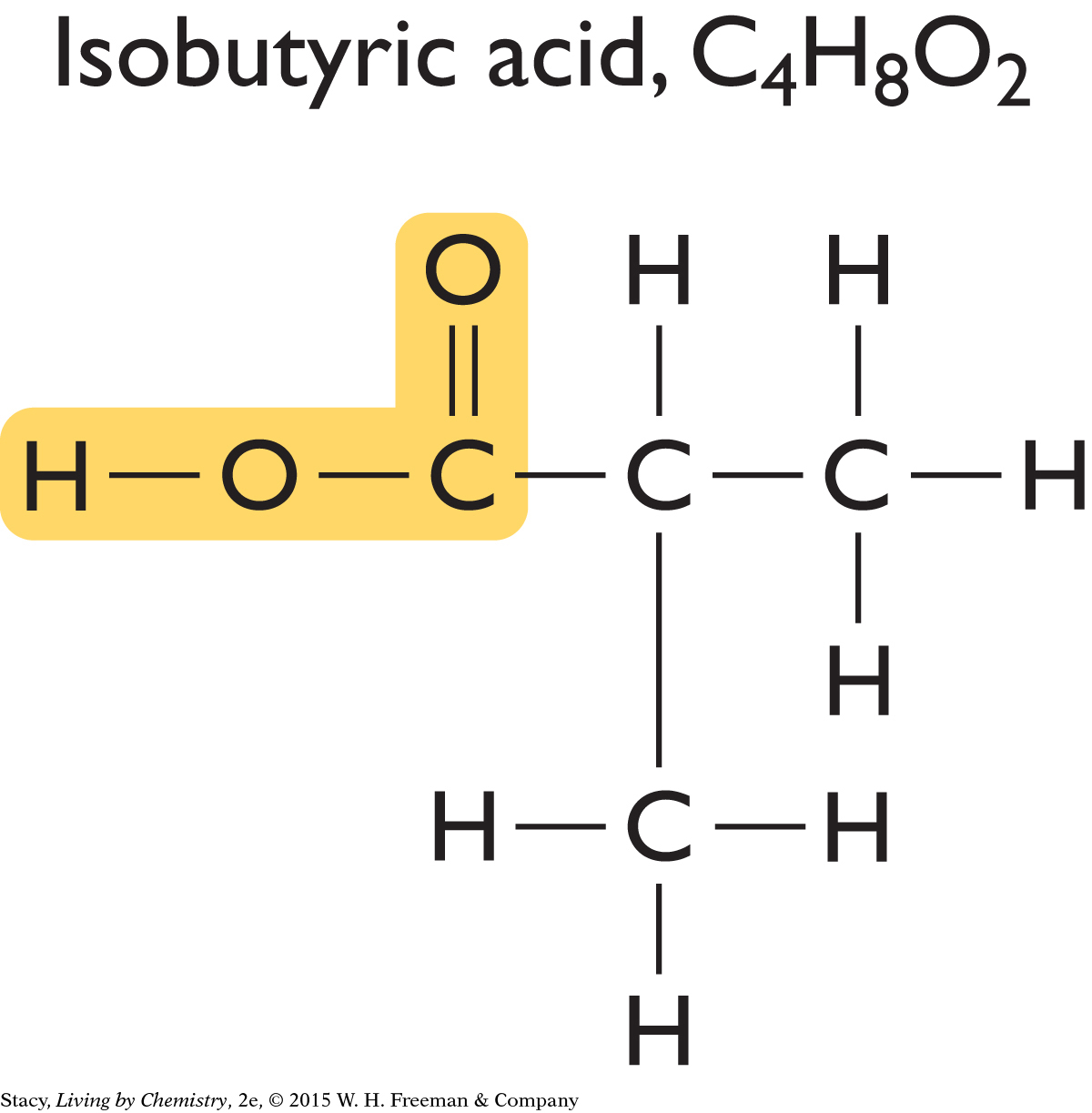
|
170
CONSUMER CONNECTION
CONSUMER
CONNECTION
The ingredients list on most of your household products reads like a chemical inventory. Examine the labels of your shampoos, cleansers, hair gels, mouthwash, and so on, to see which chemical names you recognize.

You can see that this same structural feature is present in isopentanoic acid and isobutyric acid as well.
SWEET-SMELLING MOLECULES
Now look at the structural formulas for two sweet-smelling molecules.
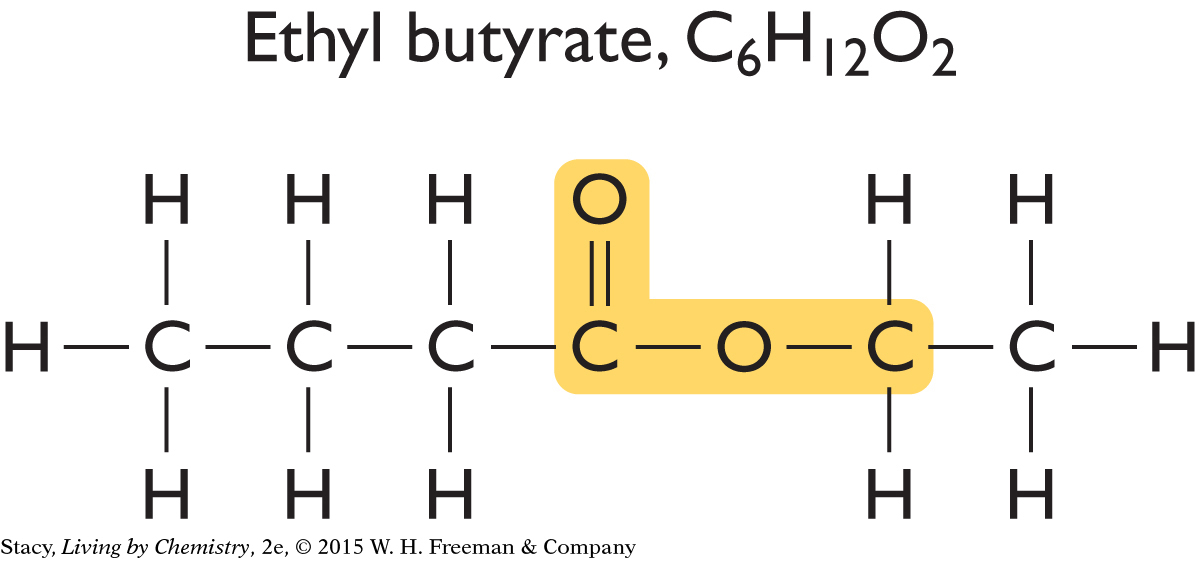
|
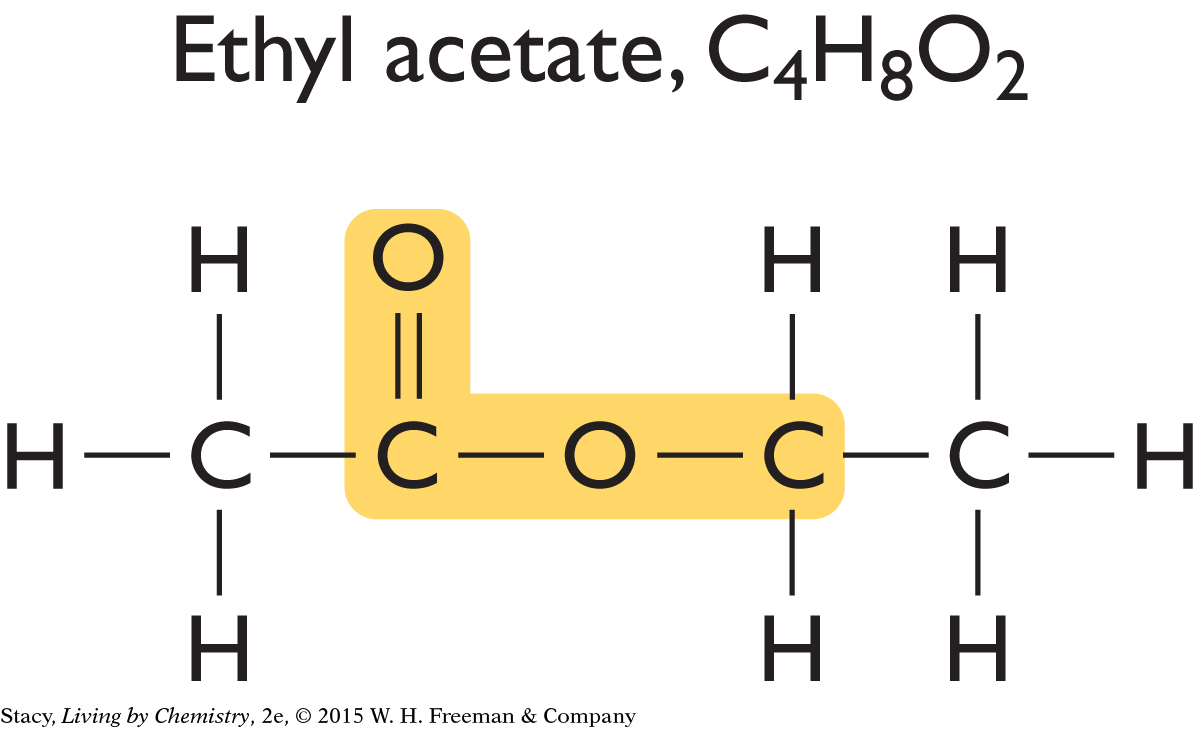
|
Just like the putrid-smelling molecules, these sweet-smelling molecules have an identical structural feature, which is highlighted in yellow. This feature is similar to the one found in putrid-smelling molecules, but it is slightly different.
Functional Groups
Functional Groups
The structural features that groups of molecules have in common are called functional groups. A functional group often stands out as an unusual or unique portion of a molecule. The functional group found in the putrid-smelling molecules is called a carboxyl functional group. The functional group found in the sweet-smelling molecules is called an ester functional group.
Two other functional groups are associated with fishy smells and minty smells.
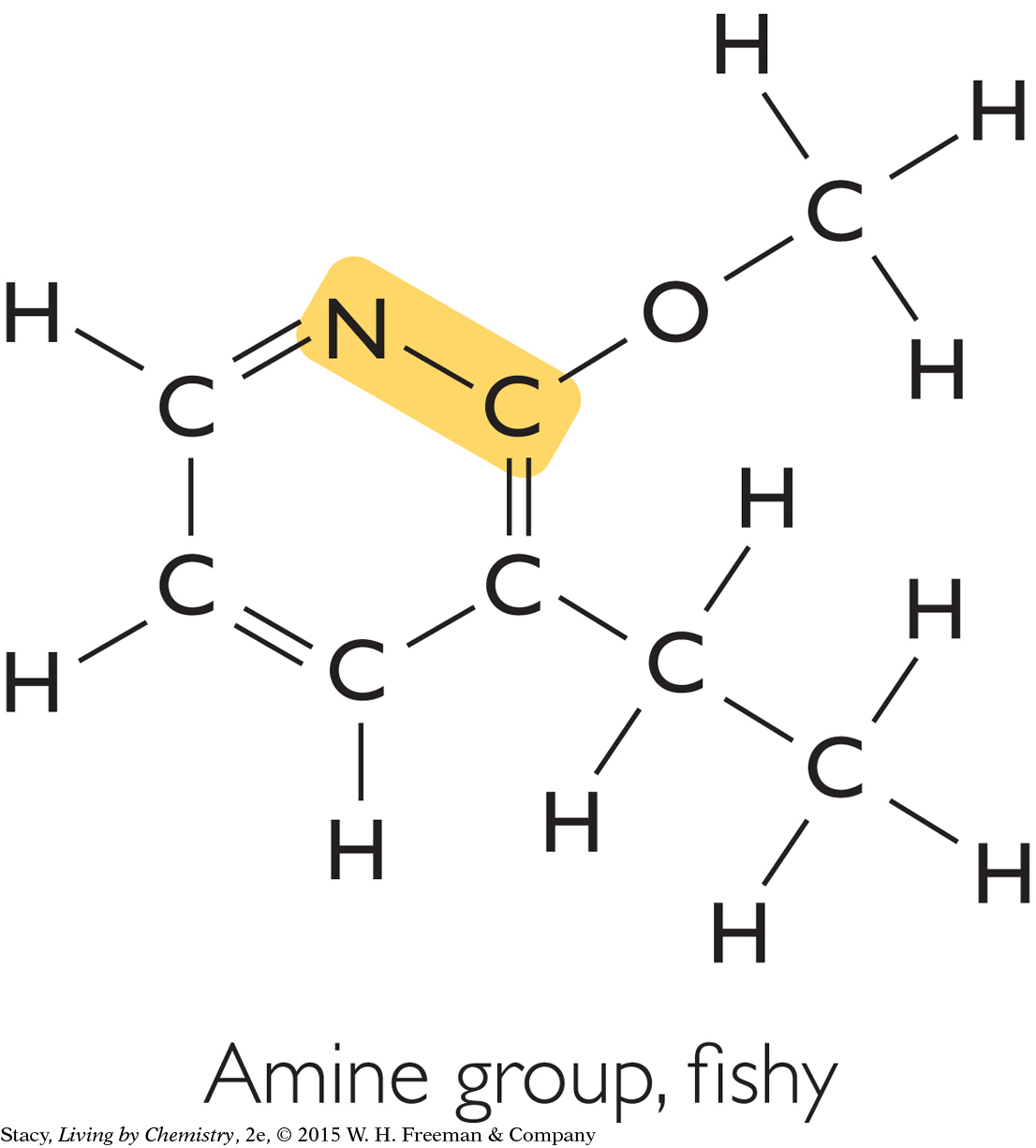
|
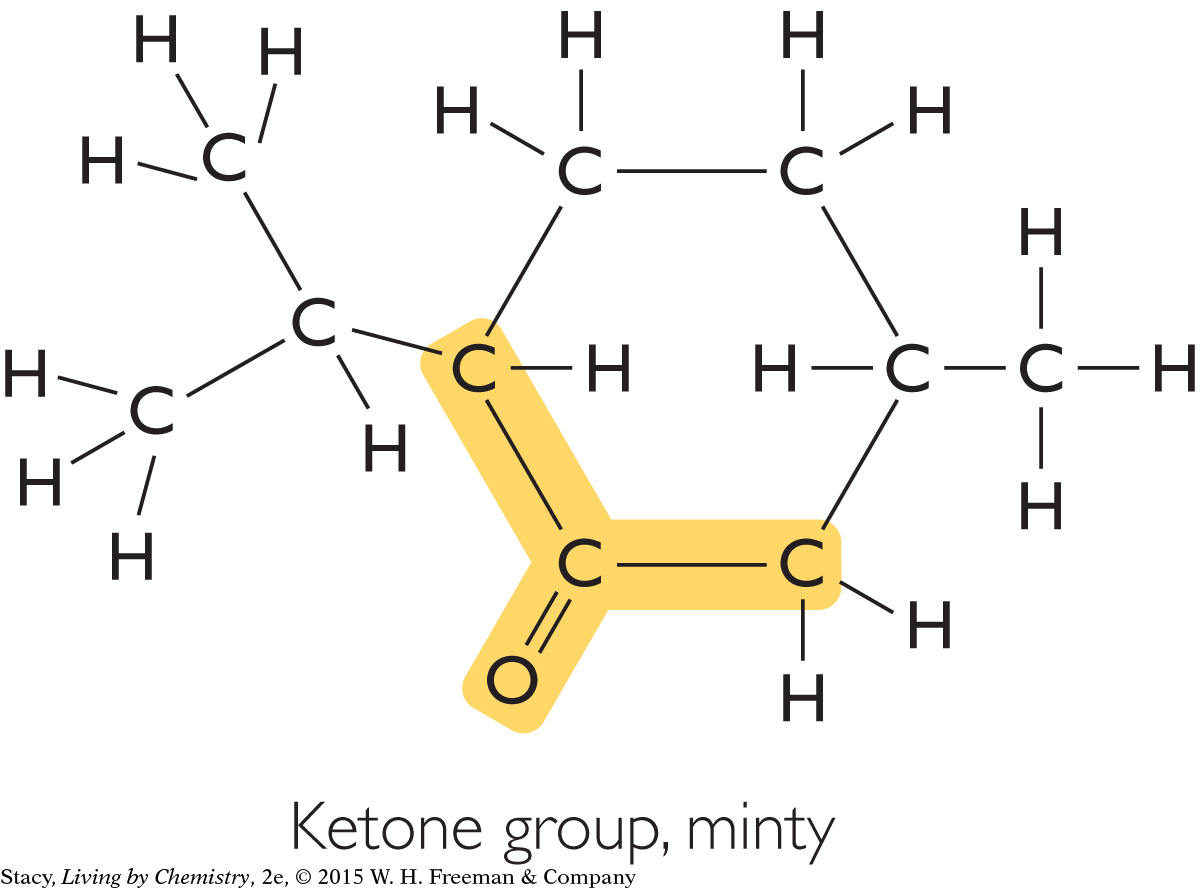
|
Example 1
Functional Group and Smell
The structural formulas of two molecules are shown here. Predict how the molecules will smell. What is your reasoning?
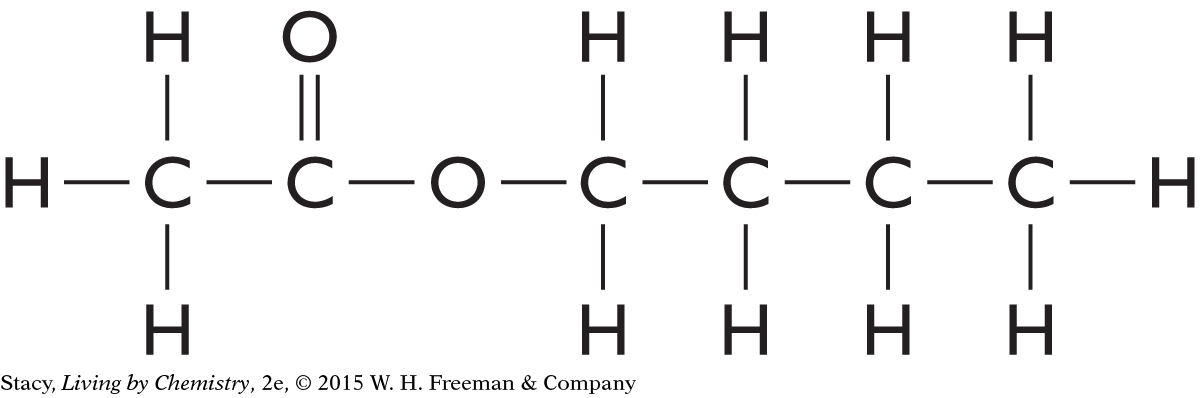
|
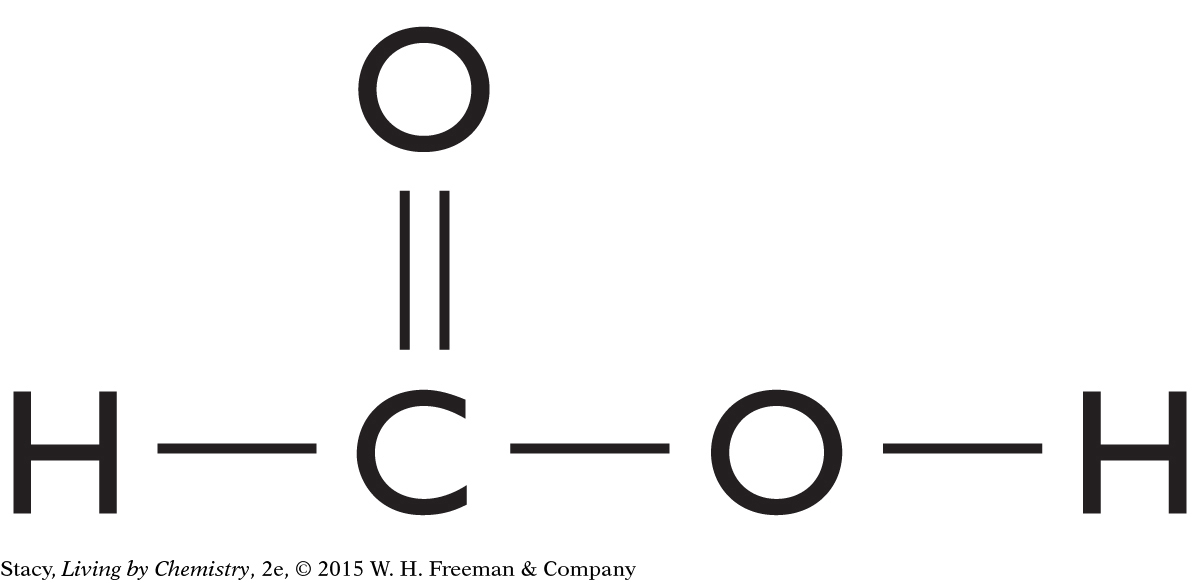
|
Solution
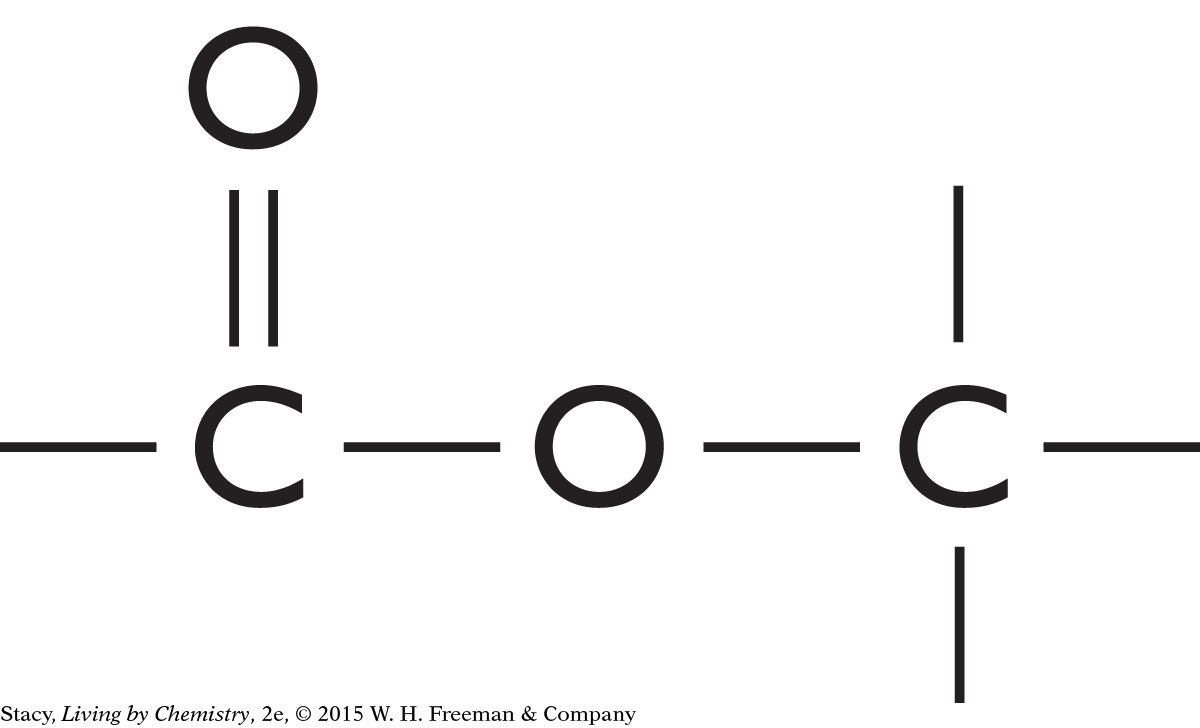
This molecule contains an ester functional group. It is probably a sweet-smelling molecule.
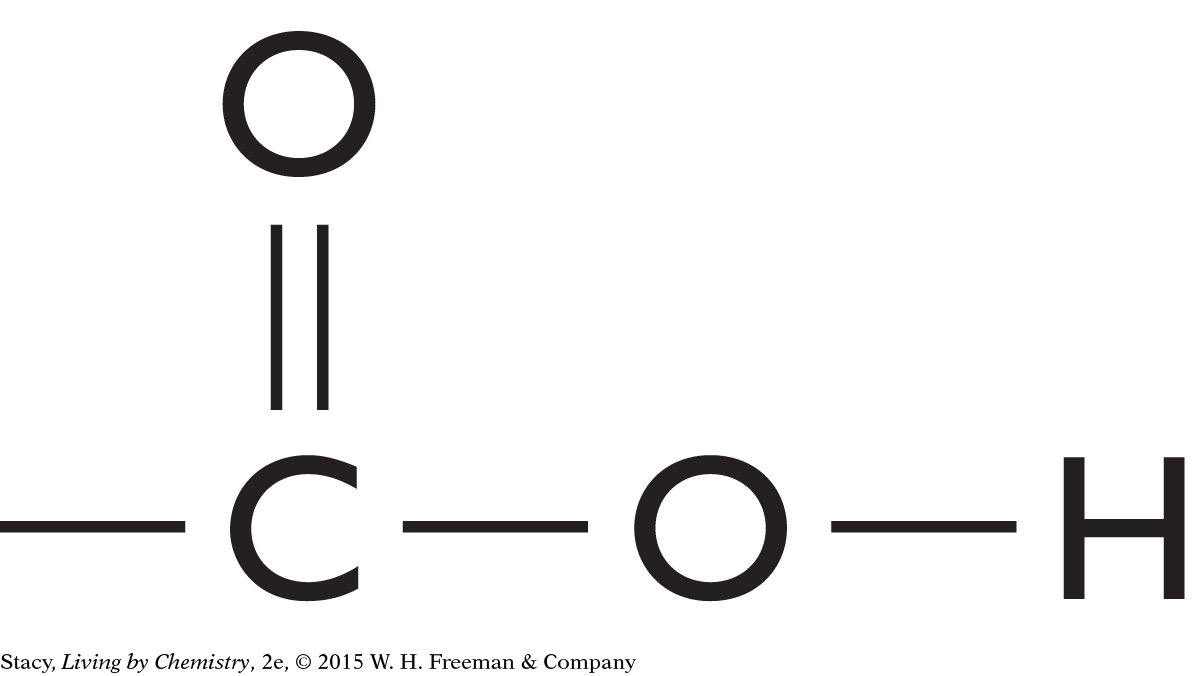
This molecule contains a carboxyl functional group. It is probably a putrid-smelling molecule.
171
OTHER FUNCTIONAL GROUPS
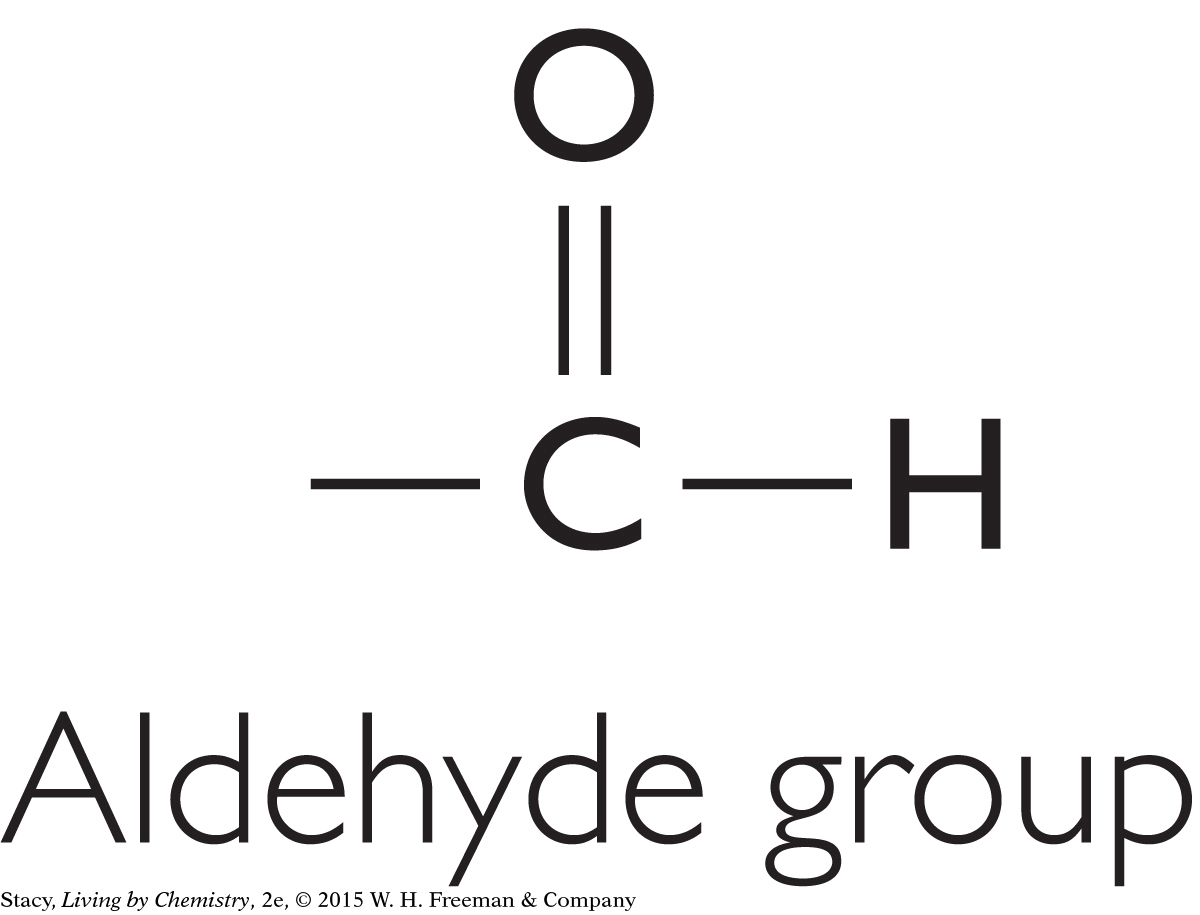
There are other functional groups besides the four discussed so far. For instance, a molecule may have a hydroxyl group (–OH). Molecules containing this feature are called alcohols. A molecule containing an aldehyde group is similar to a ketone, except that the carbon that is double bonded to the oxygen is between a carbon and a hydrogen.
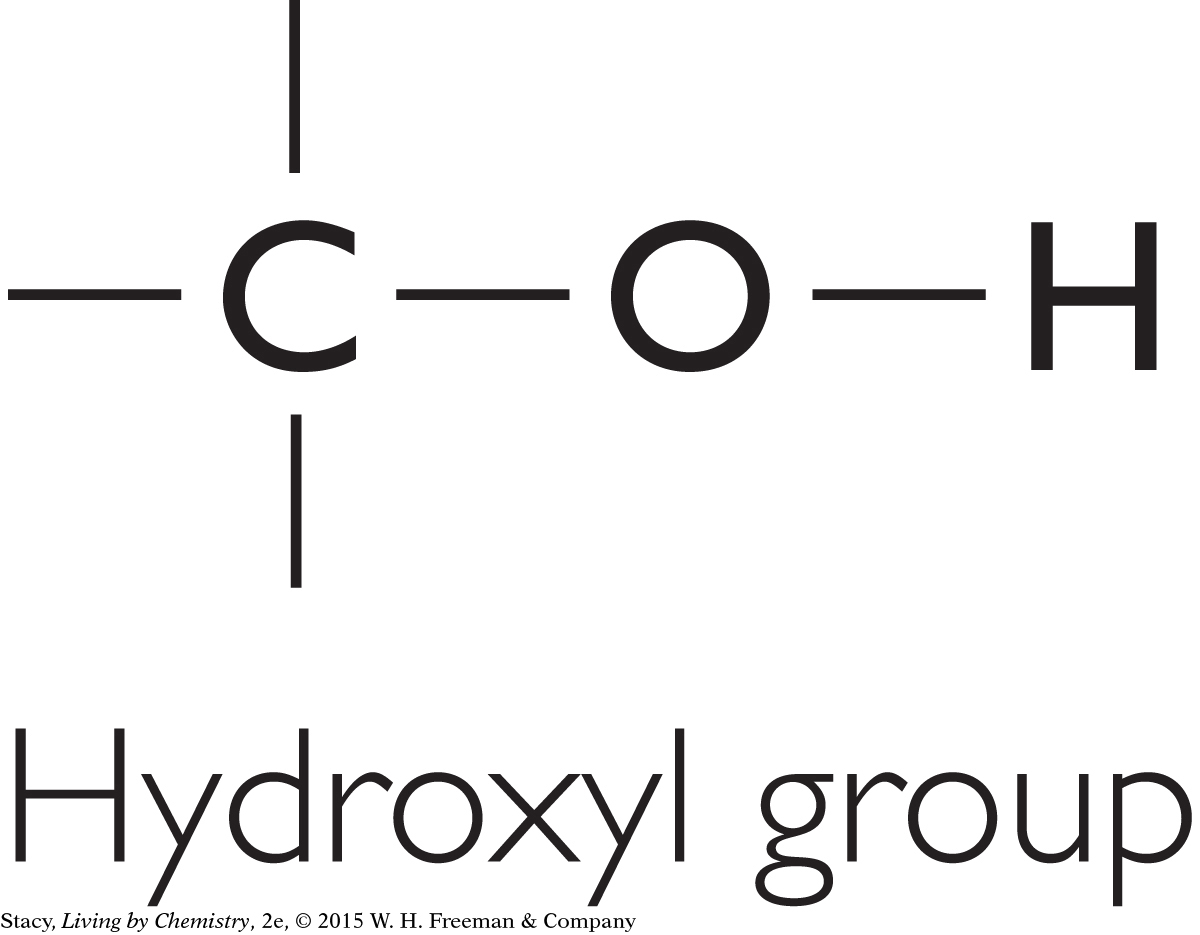
|
|
Another type of molecule is one containing a single oxygen located between two carbon atoms. Molecules containing this feature are called ethers.
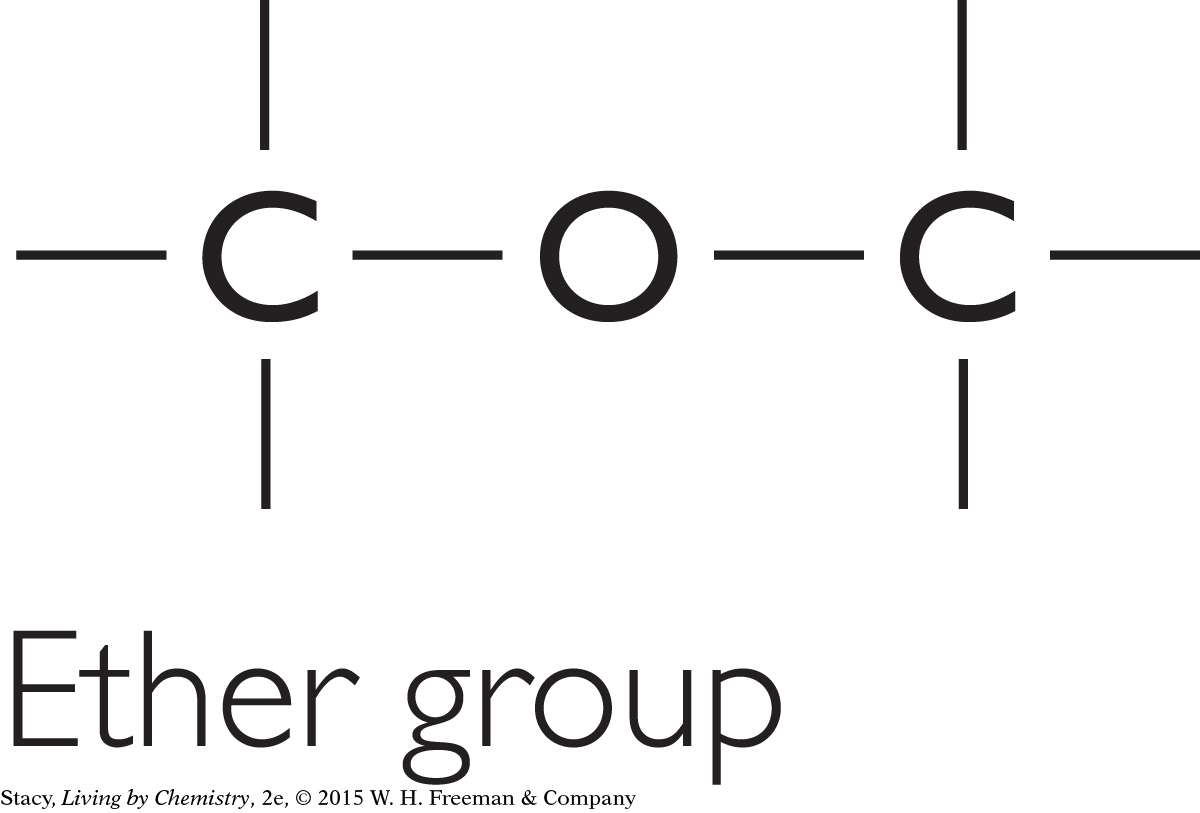
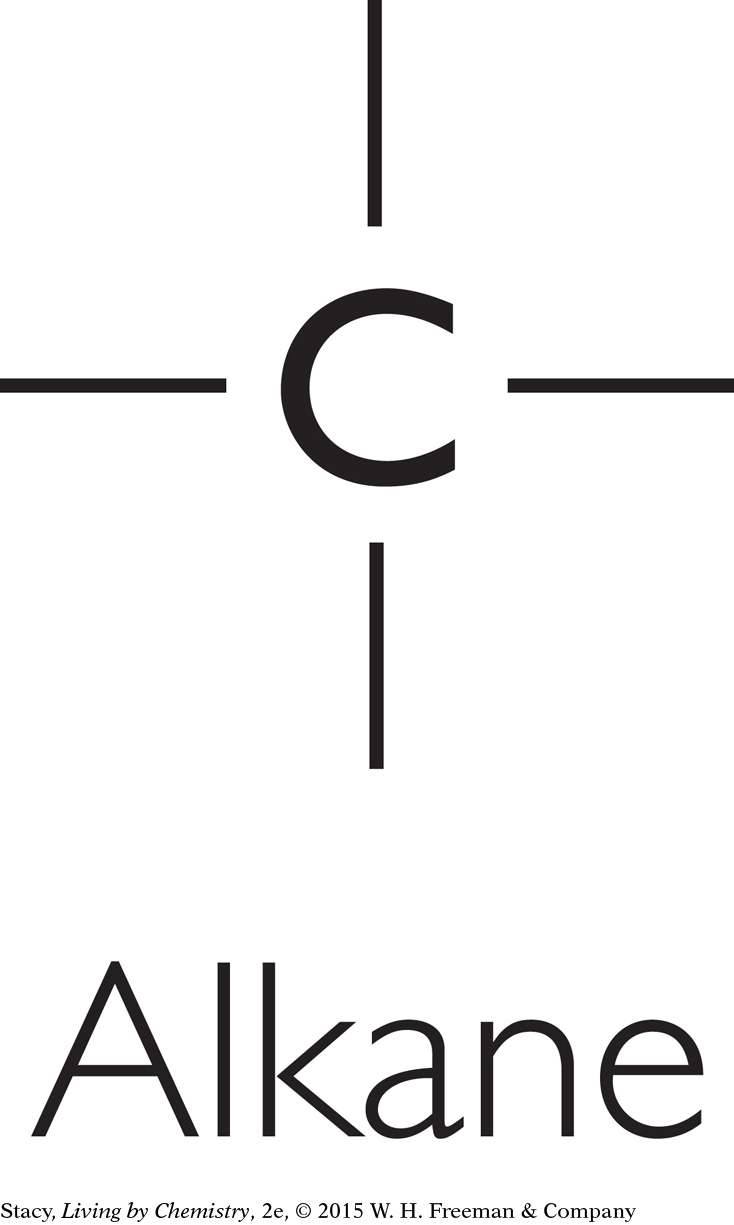
Some molecules have no functional groups. For example, alkanes consist of only carbon and hydrogen atoms connected with single bonds.
Classifying Molecules
Classifying Molecules
The easiest way to classify molecules is by functional group. All the molecules that have ester functional groups are referred to as esters. All the sweet-smelling compounds you have encountered so far contain an ester functional group and have two-word names that end in “-yl” and “-ate.” Ethyl butyrate, ethyl acetate, and hexyl acetate all smell sweet.
The putrid-smelling compounds have a carboxyl group and names that end with “-ic acid.” Butyric acid and hexanoic acid both smell putrid.
You may be able to identify the smell of a molecule by paying attention to its chemical name. Examine the compounds in the table. Each of these molecules has a unique smell even though they all have three carbon atoms and at least six hydrogen atoms. Their names are also very different.
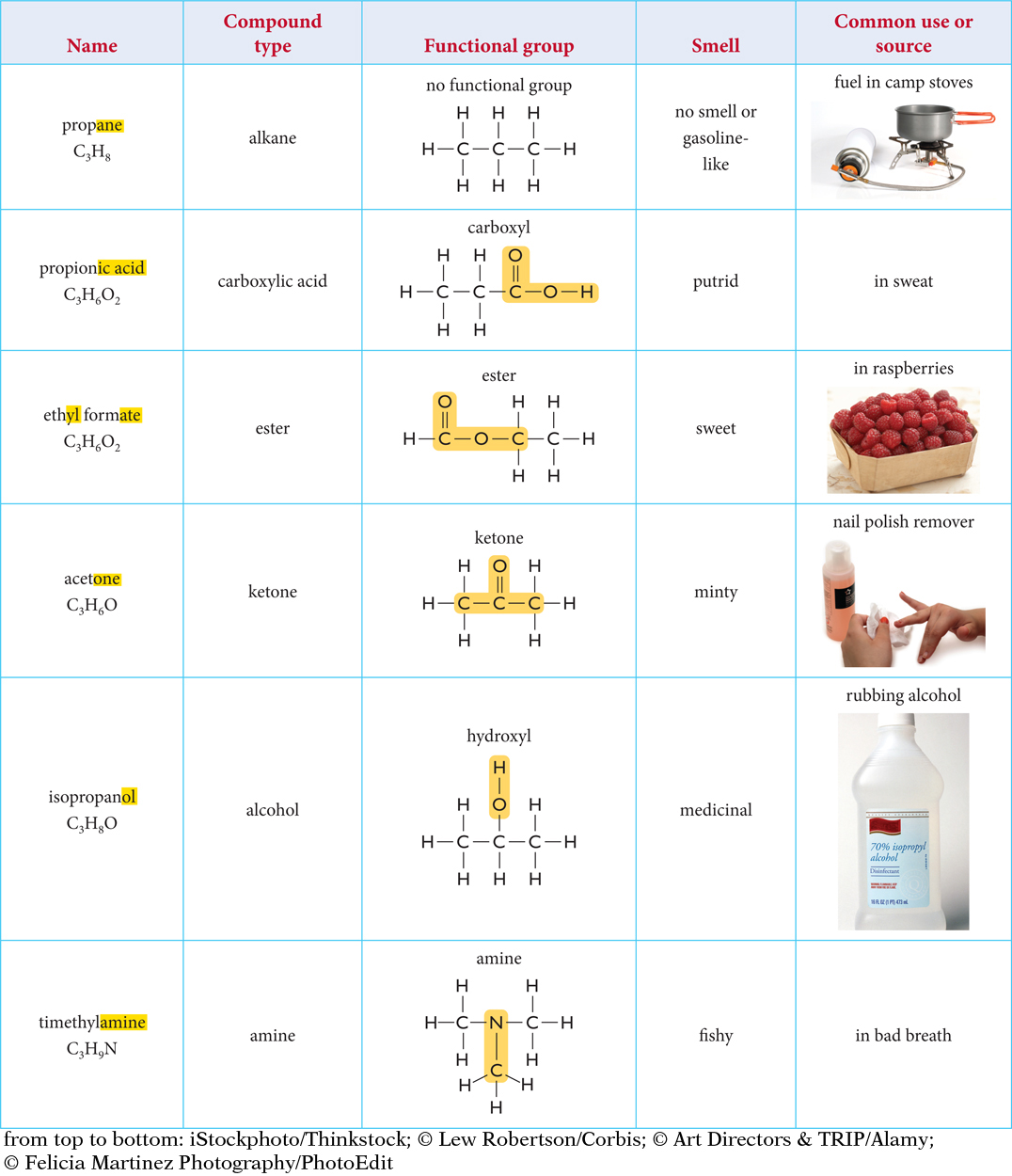
172
Compounds that have the same functional group tend to have similar properties. For example, esters smell sweet, dissolve in water, and change phase from liquid to gas fairly easily. There are exceptions. For example, larger alkanes tend to have a gasoline smell, while smaller alkanes like propane in the table have almost no smell.
LESSON SUMMARY
LESSON SUMMARY
What does the structure of a molecule have to do with smell?
KEY TERM
functional group
Based on what the lessons have covered so far, compounds that have similar smells also appear to have similar structural features. These features are called functional groups. Functional groups have names. Compounds are frequently named according to the functional groups they contain. If you identify the functional group in a compound, you may also be able to predict how that compound will smell.
173
Exercises
Reading Questions
What is a functional group?
What information would you want to have to predict the smell of a compound? Explain why.
Reason and Apply
Explain why C2H4 has fewer hydrogen atoms than C2H6.
Create a four-carbon molecule that is
an alkane
a carboxylic acid
an alcohol
an ester
Consider a compound called hexanol. Chemists can tell from its name that it has six carbons (hex-) and it is an alcohol (-ol).
What is the molecular formula of hexanol?
Draw a possible structural formula for hexanol.
Is the molecular formula or the structural formula more useful in determining the smell of hexanol? Explain.
Research Examine the ingredients lists on some household products, such as shampoo, lotion, or cleanser. Write down the names of compounds and functional groups that you were able to identify.
If you were a chemist and you wanted to invent a new smell, what would you think about doing or creating?
If you were a chemist and you wanted to change the smell of a molecule, what might you try to do to that molecule?
Explain what you think is going on in this cartoon.
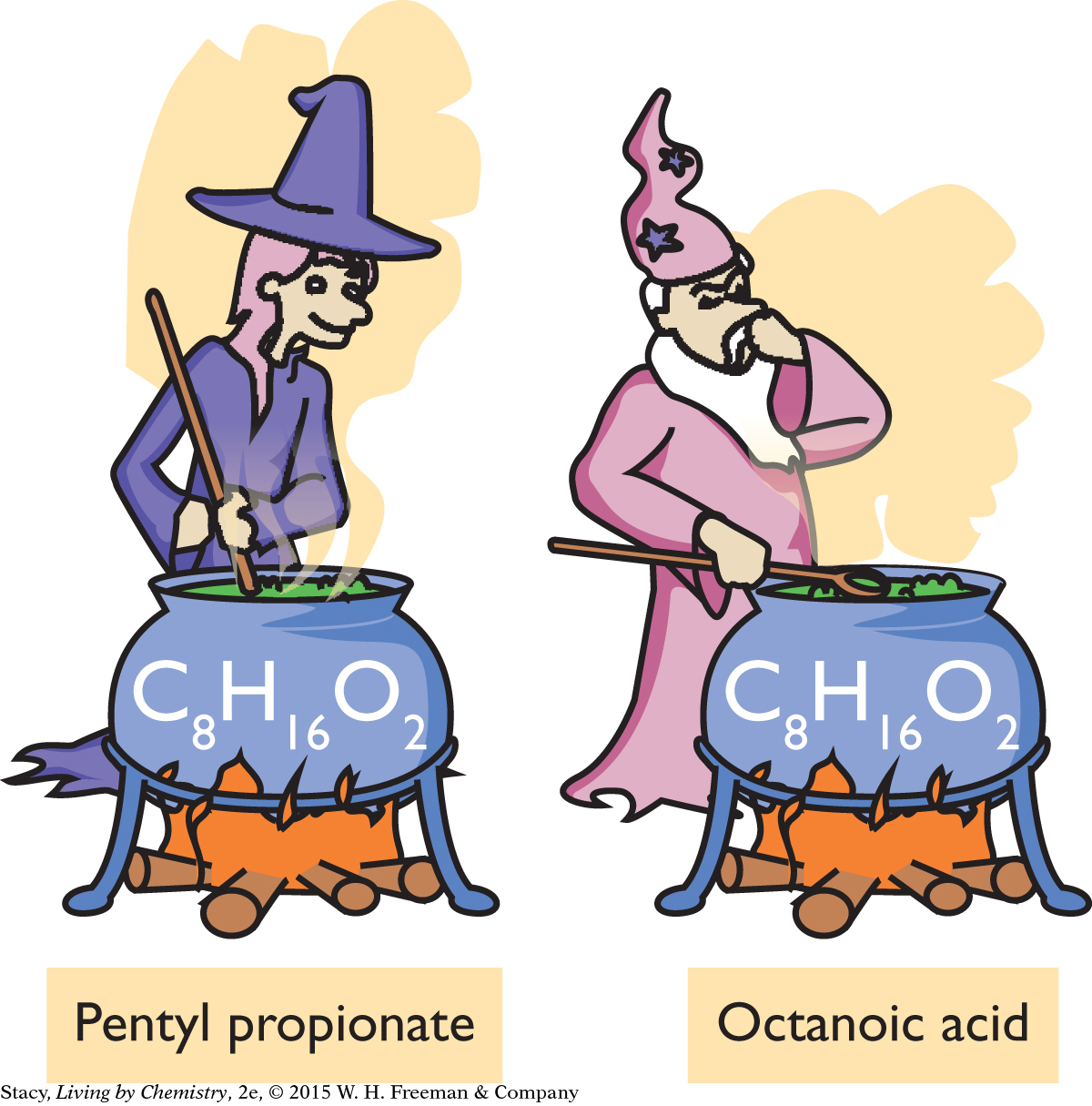
174
Structural formulas for six molecules are shown here. Identify (by name) the functional group in each molecule.
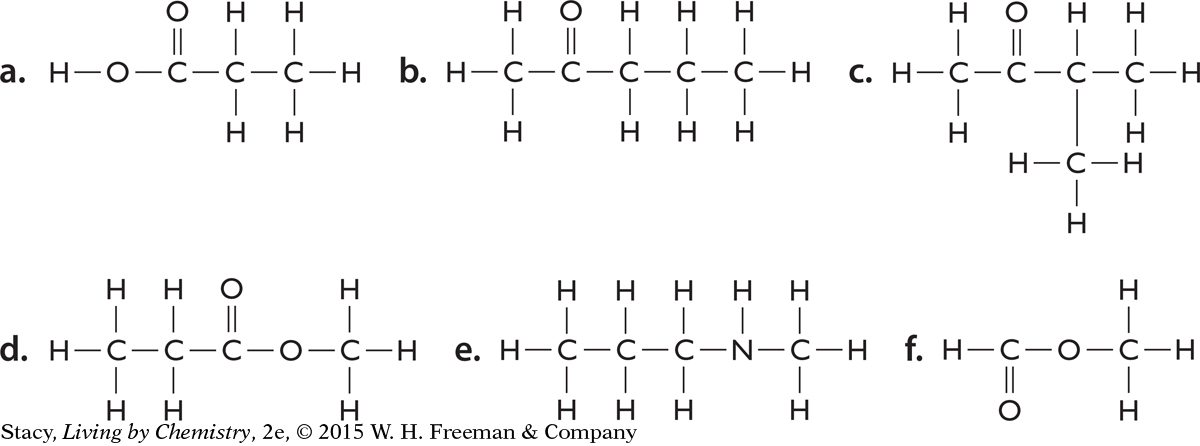
Write the molecular formula for each of the molecules in Exercise 10.