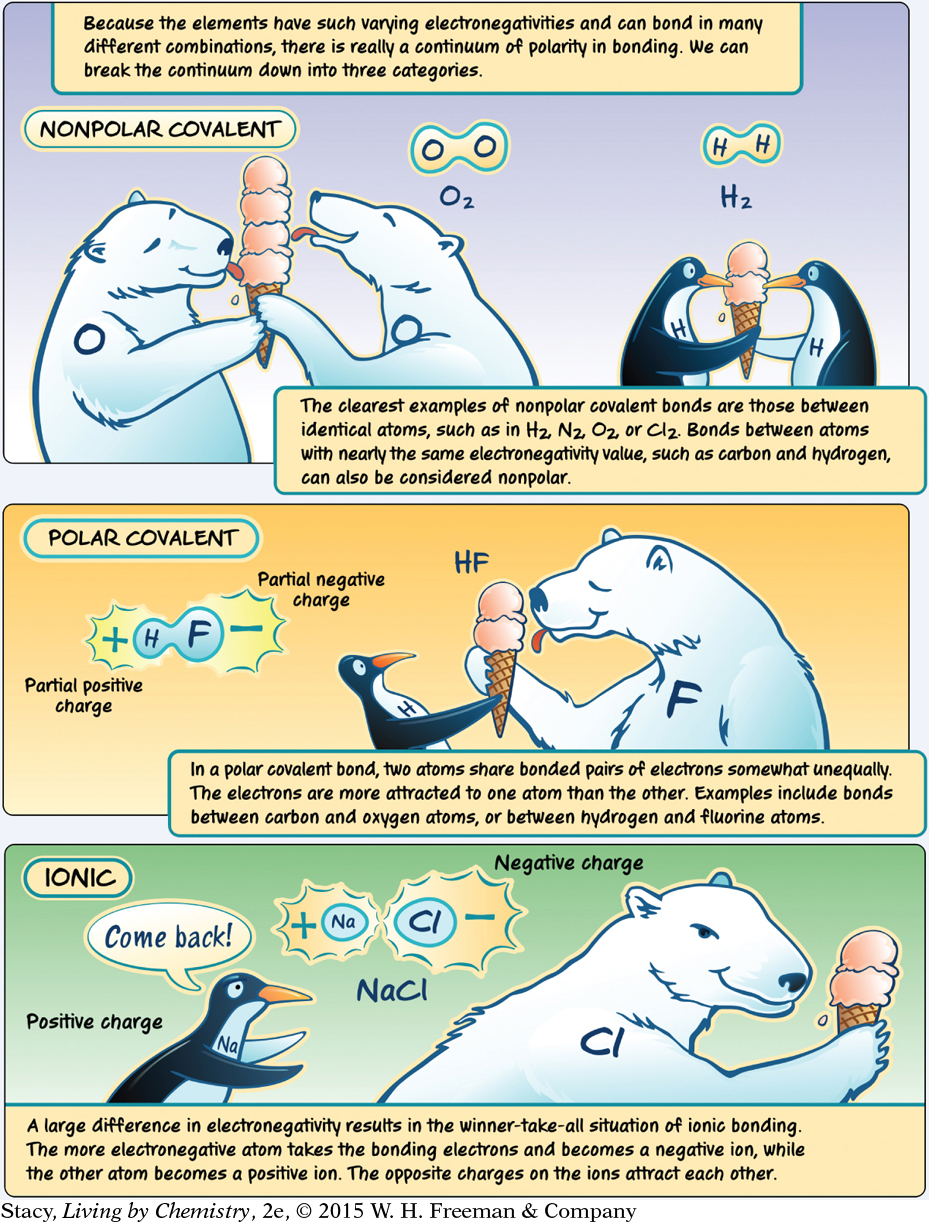
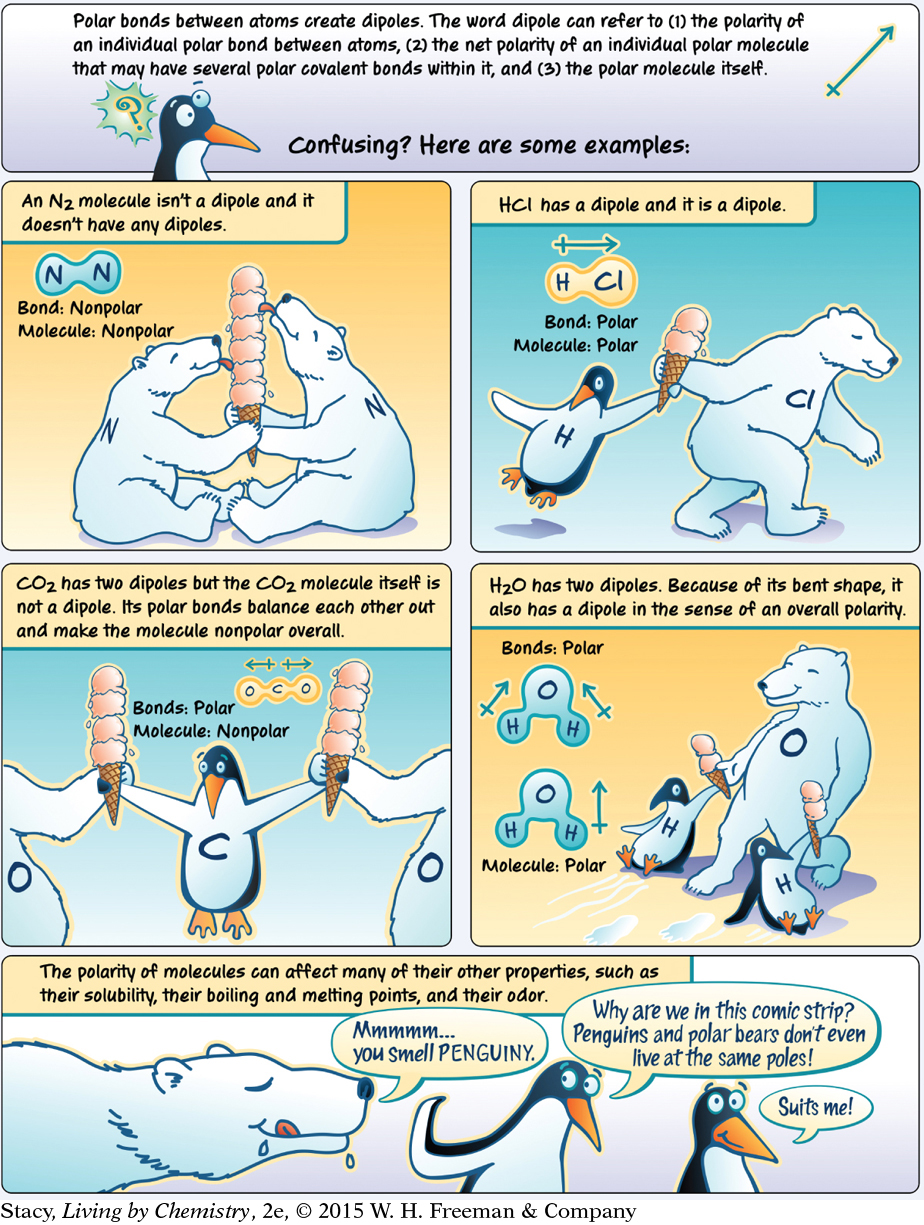
LESSON 42: Attractive Molecules: Attractions between Molecules
THINK ABOUT IT
Not everything has a smell. In fact, there are more substances on the planet that we don’t smell than substances that we do smell. If everything around us had a smell, our noses would probably be overwhelmed most of the time.
Why do some molecules smell while others do not?
To answer this question, you will explore
Compounds That Do Not Have a Smell
Polar Molecules
Intermolecular Force
Compounds That Do Not Have a Smell
EXPLORING THE TOPIC
Compounds That Do Not Have a Smell
Take a big whiff of air. Perhaps you are able to detect some odors—a freshly mowed lawn, somebody’s food cooking. But the air itself does not smell. Small molecules, such as nitrogen, oxygen, carbon dioxide, and water vapor, obviously fit inside the receptor sites. So, why can’t we smell them?
Perhaps these molecules are too small to stay docked in a receptor site. But some small molecules do have a smell. Hydrogen sulfide, H2S, smells like rotting eggs and ammonia, NH3, smells pungent.
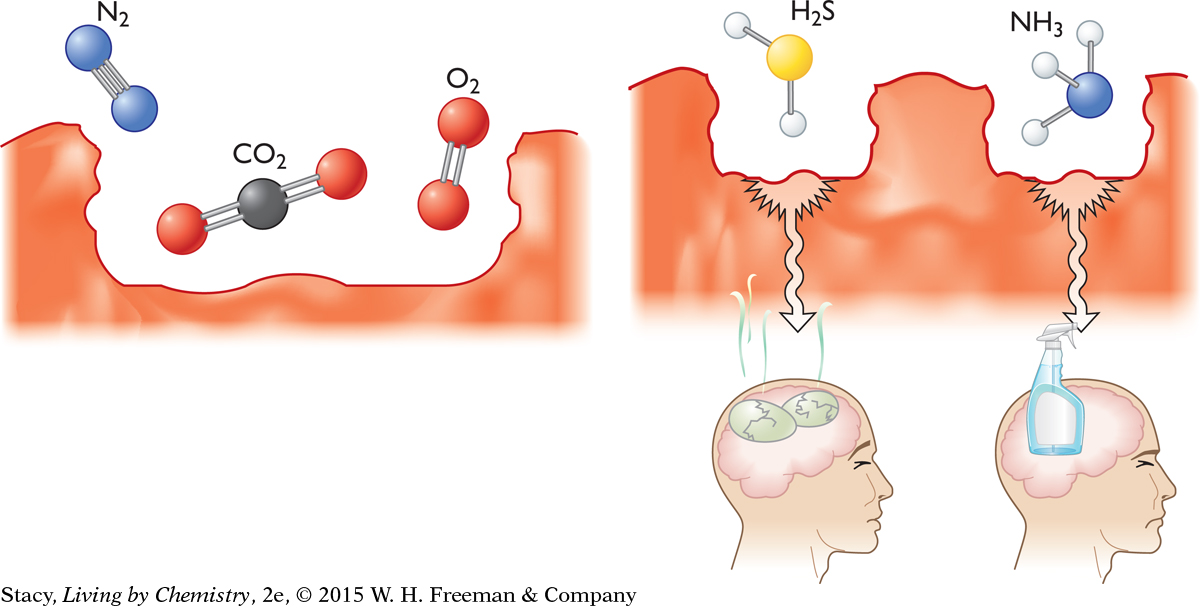
So the molecular size of a compound does not explain whether we can smell it. It turns out that a molecular property called polarity is involved.
Polar Molecules
Polar Molecules
PHYSICS CONNECTION
PHYSICS
CONNECTION
You take off a wool hat, and all your hair stands on end. This happens because electrons move from your hair to the hat so that each individual hair has a positive charge. Because like charges repel one another, the hairs try to get as far apart as possible, resulting in a bad hair day. Something similar happens when a child slides down a plastic slide.

When two different materials are rubbed together, some electrons can transfer from one material to the other. The result is an imbalance of positive and negative charges. One material has an excess of positive charge and the other an excess of negative charge. This is known as static electricity.
Suppose that you rub a plastic wand on a piece of cloth. The plastic wand will end up with a charge. In class you tested a number of liquids by holding a charged wand near them and observed that some of the streams bend toward the wand.
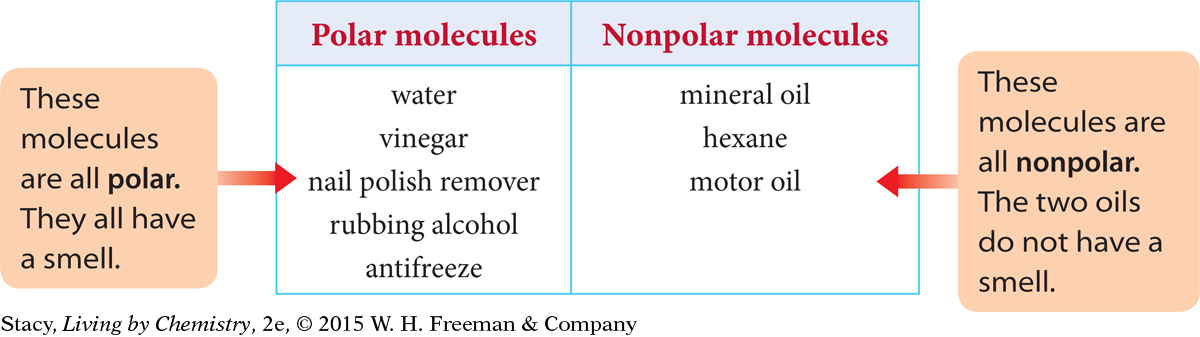
In this table, all of the liquids in the first column are attracted to a charged wand. They are made up of polar molecules. The liquids in the second column are not attracted to a charged wand. They consist of nonpolar molecules.
Water is a polar molecule. As this illustration shows, there are partial charges at different locations on the water molecule. These partial charges are much smaller than the charge on an individual electron or proton. However, the charges are large enough to cause a stream of water to be attracted to a charged wand.
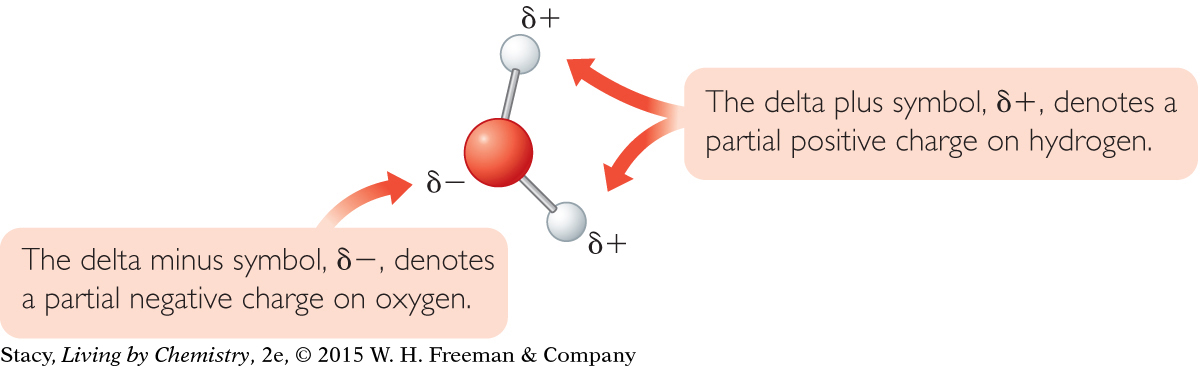
The oxygen atom has a partial negative charge, and the hydrogen atoms each have a partial positive charge.
Intermolecular Force
Intermolecular Force
EXPLAINING THE CHARGED WAND

When a wand with a negative charge is placed next to a stream of water, the water molecules orient themselves in space so that their positive ends are lined up in the direction of the wand. As a result, the whole stream of water is attracted to, or pulled toward, the charged wand. The illustration on the next page shows a possible way to depict what happens to the molecules.
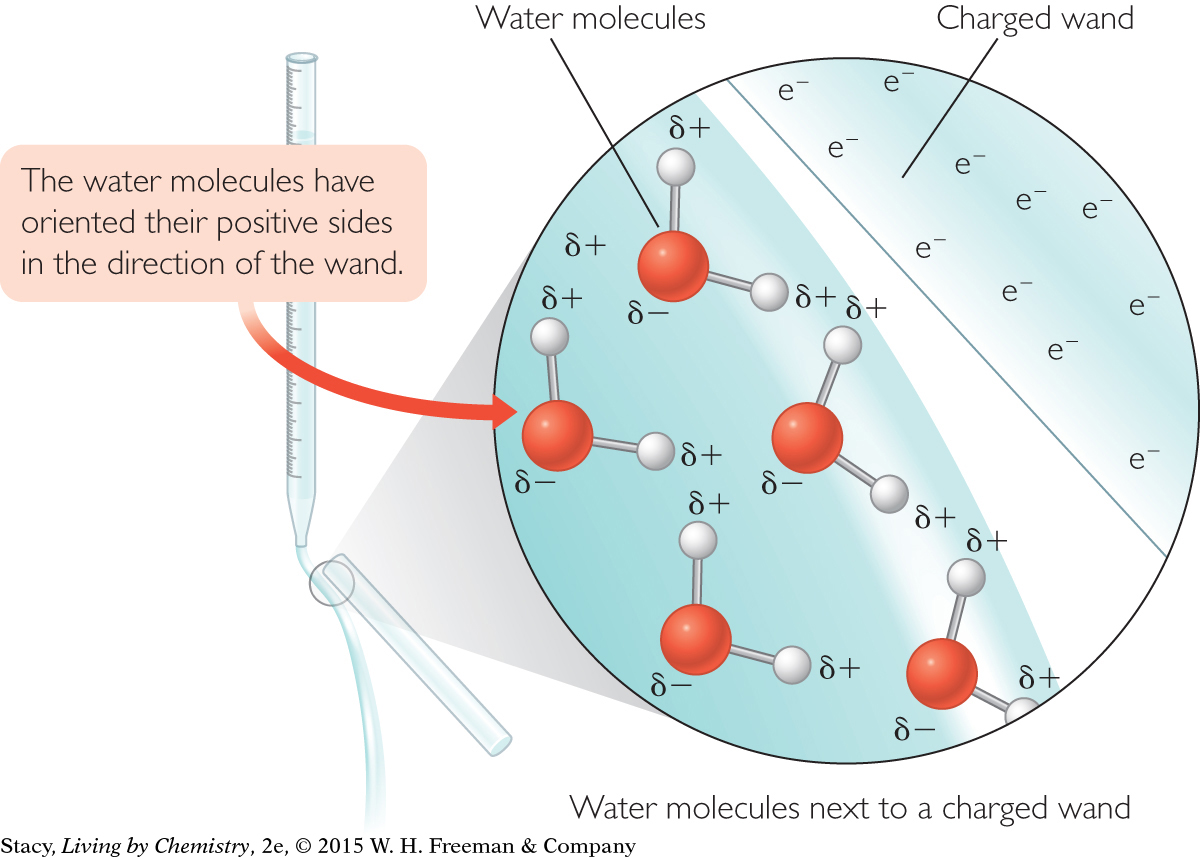
INTERMOLECULAR BEHAVIOR
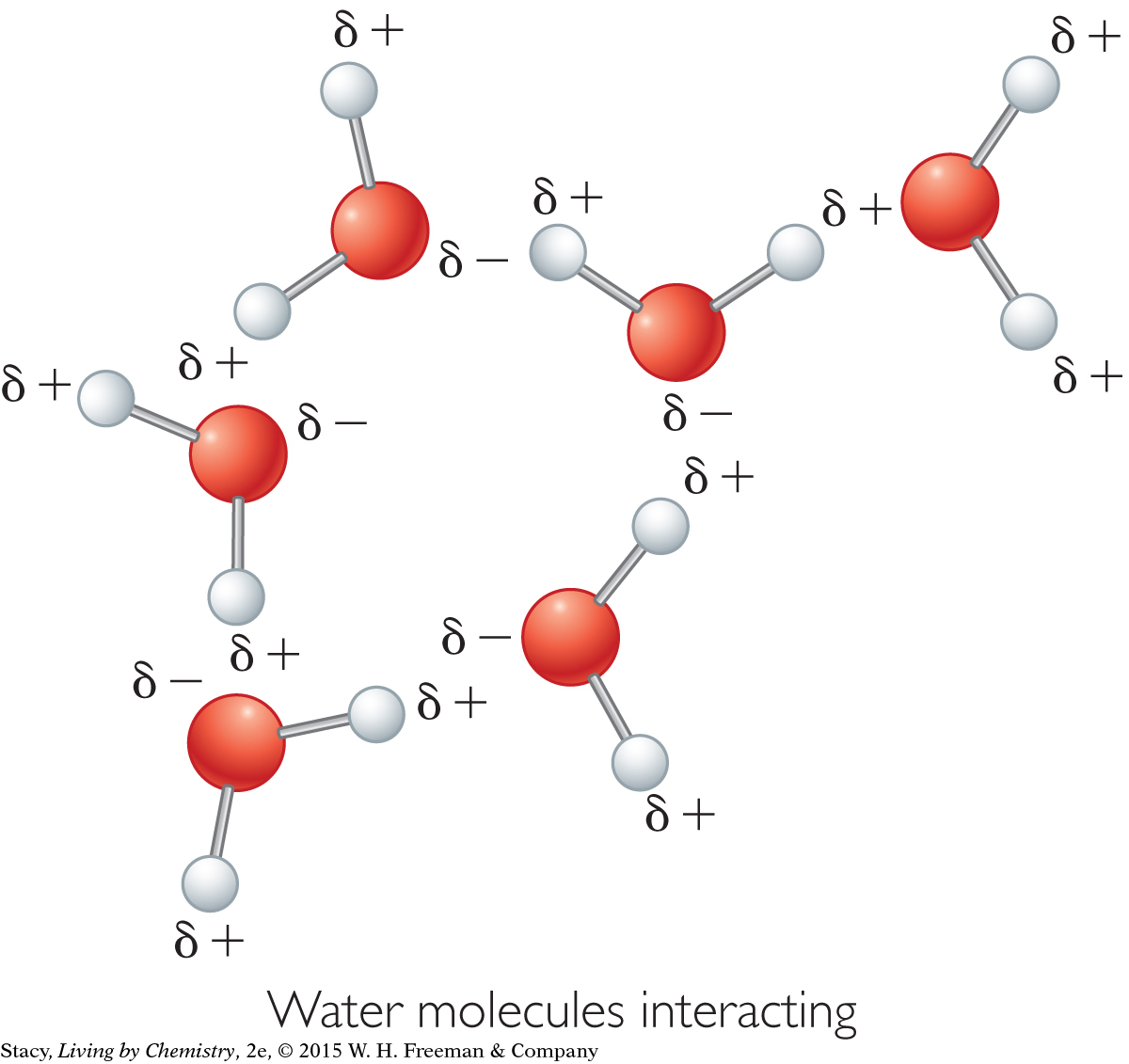
The partial charges on polar molecules cause more than an attraction to a charged wand. They also cause attractions between molecules. As the molecules in a polar liquid tumble around, they tend to align with each other because partial negative charges are attracted to partial positive charges. This attraction between individual molecules is an intermolecular force. The prefix inter- means “between” and the force is a force of attraction.
Intermolecular attractions can be used to explain many observable properties.
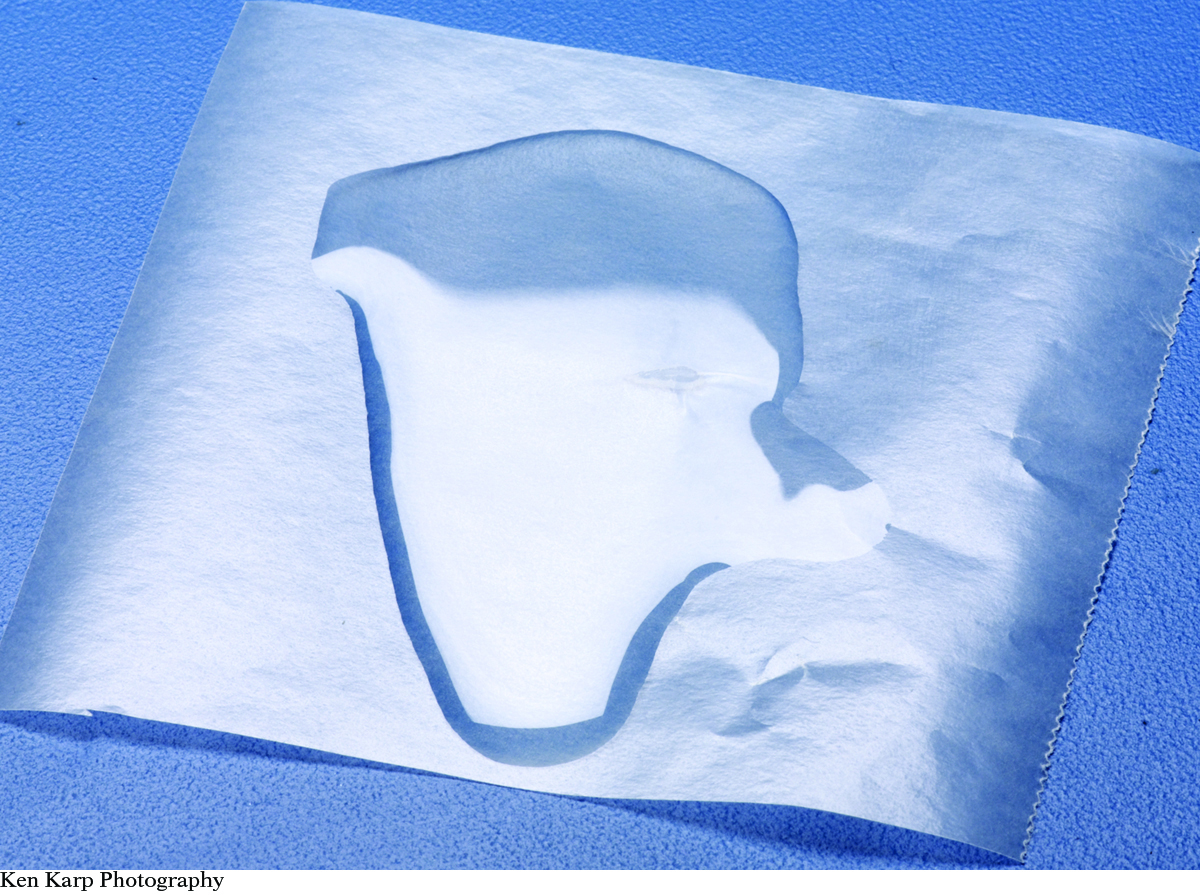
Observation: Water beads up on waxed paper. Oil spreads out.

Ken Karp Photography
|
Ken Karp Photography
|
LANGUAGE CONNECTION
LANGUAGE
CONNECTION
The saying “Oil and water don’t mix” is one way to remember that a nonpolar substance and a polar substance tend not to dissolve in each other.

Explanation: The water molecules are polar, while the oil molecules are nonpolar. The individual water molecules are attracted to each other and “cling together.” Individual oil molecules are not attracted to one another to the same extent, and they spread out.
Observation: Water is a liquid at room temperature, but methane is a gas at room temperature. They have roughly the same molecular mass.
Explanation: Water is polar and methane is nonpolar. The individual water molecules are attracted to each other and stay together as a liquid. The attractions between methane molecules are much weaker. The methane molecules spread throughout the room as a gas.
Observation: Methanol dissolves easily in water. Oil floats on top of water but does not dissolve.
Explanation: The polar methanol molecules are attracted to the polar water molecules and go into solution. The nonpolar oil molecules are not attracted to the polar water molecules.
LESSON SUMMARY
LESSON SUMMARY
Why do some molecules smell while others do not?
KEY TERMS
polar molecule
nonpolar molecule
partial charge
intermolecular force
Molecules can be divided into two classes: polar and nonpolar. Polar molecules are attracted to a charged wand because they have partial charges distributed within the molecule. Nonpolar molecules are not attracted to a charged wand. Polarity is responsible for intermolecular attractions that affect many properties of molecules, possibly including smell properties.
Exercises
Reading Questions
Explain in your own words what a polar molecule is.
What are intermolecular attractions?
Reason and Apply
Lab Report Write a lab report for the Lab: Attractions Between Molecules. In your conclusion, explain why some liquids were attracted to the wand and others were not, and why some liquids beaded up on waxed paper while others did not.
 Page 218
Page 218Methanol, CH3OH, is attracted to a charged wand. The hydrogen atoms have a partial positive charge and the oxygen atom has a partial negative charge.
Draw a picture showing how you predict the methanol molecules are oriented when they are attracted to a wand with negative charges.
Do you expect methanol to bead up or spread out on waxed paper? Explain your thinking.
Do you expect methanol to dissolve in water? Explain your thinking.
Hexane, C6H14, is not attracted to a charged wand and it spreads out on waxed paper. Do you expect hexane to dissolve in water? Explain your thinking.
Explain why no liquids are repelled from a charged wand.