LESSON 44: Thinking (Electro)Negatively: Electronegativity Scale
227
THINK ABOUT IT
Some atoms are “greedier” than other atoms when it comes to sharing the bonding electrons between them. A bond between two atoms with very different electronegativities is more polar than a bond between atoms with similar electronegativities. How can the polarity of different bonds be compared?
How can electronegativity be used to compare bonds?
To answer this question, you will explore
Electronegativity Scale
Diatomic Molecules
Electronegativity Scale
EXPLORING THE TOPIC
Electronegativity Scale
Chemists have assigned each atom a number, called an electronegativity value. This number corresponds to the tendency of an atom to attract bonding electrons. By using these numbers, it is possible to compare the polarity of different bonds. The table here shows the electronegativity value for an atom of each element in the periodic table.
HISTORY CONNECTION
HISTORY
CONNECTION
Linus Pauling, inventor of the electronegativity scale, is one of only two people (the other is Marie Curie) to receive Nobel Prizes in two different fields. In 1954, he received the Nobel Prize in Chemistry, and in 1962 he received the Nobel Peace Prize for his work in campaigning against above-ground nuclear testing.

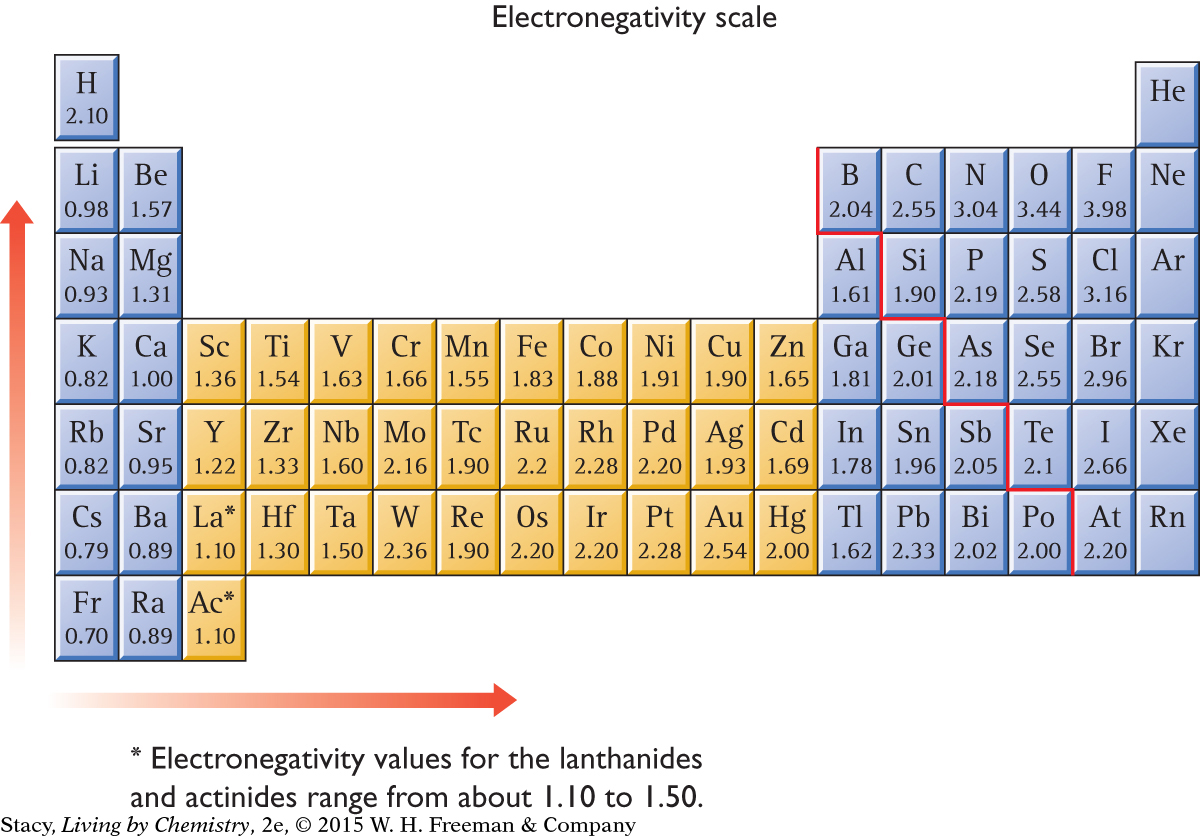
Notice that the values generally increase from left to right and bottom to top. Noble gases are often not assigned values because they generally do not form compounds.
228
A scale for electronegativity was first proposed by Linus Pauling in 1932. The scale chemists use today ranges in value from 4.0 down to 0. The electronegativity scale allows you to compare individual atoms. For example, fluorine, with an electronegativity value of 3.98, attracts shared electrons more strongly than hydrogen, with an electronegativity value of 2.10.
Diatomic Molecules
Diatomic Molecules
The electronegativity scale also allows you to compare the polarity of bonds. In a polar covalent bond, the electrons tend to spend more time around the more electronegative atom. Several substances with only two atoms are shown below. Molecules with two atoms are called diatomic molecules. By looking at the numerical difference between the electronegativities of the two atoms, it is possible to compare the polarity of one bond to another. Bonds that have a greater difference in electronegativity between the two atoms are more polar.
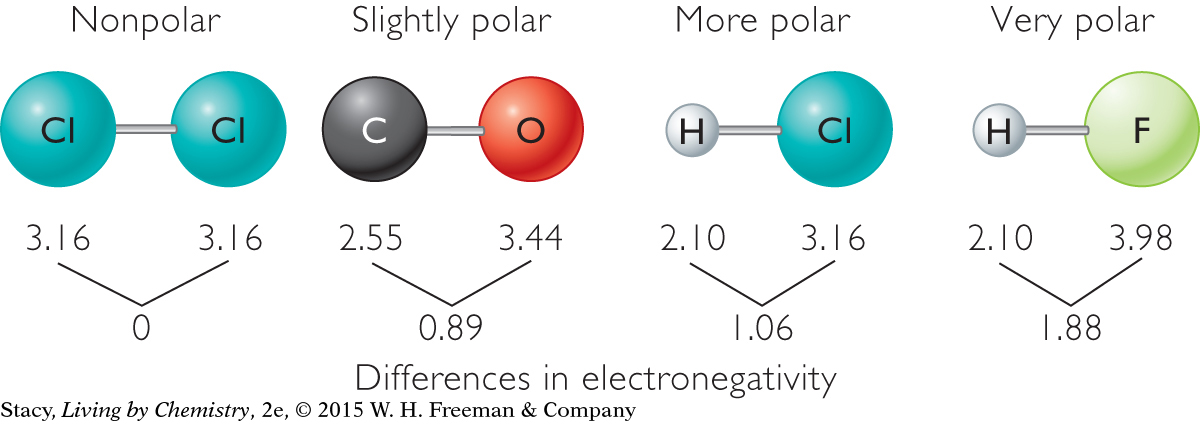
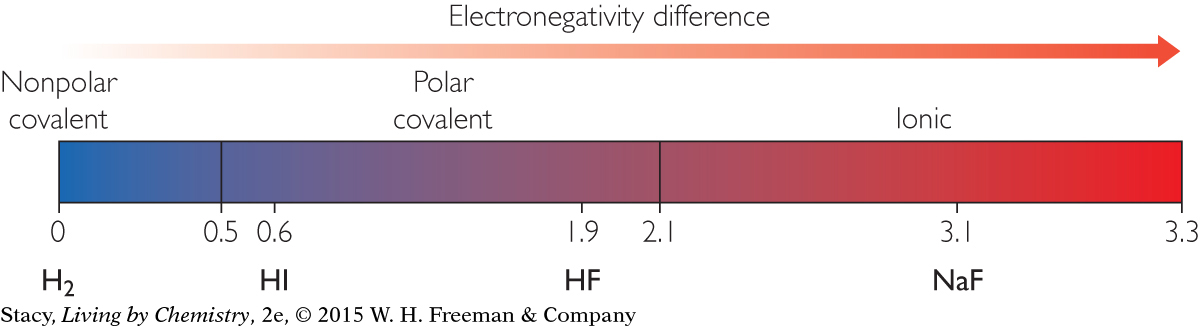
When the difference in electronegativity between the two atoms is very large, the bond is no longer considered covalent. In that case, the electrons are transferred from the less electronegative atom to the more electronegative atom. A cation and an anion form that are attracted to one another in an ionic bond. As shown on this electronegativity scale, when the difference between the electronegativities of two bonded atoms is greater than about 2.1, the bond is considered ionic:
Example
Potassium and Chlorine
Predict the type of bond you would find between potassium and chlorine.
Solution
Use the electronegativity scale to find the electronegativities of potassium, K, and chlorine, Cl. It shows that the values are 0.82 for K and 3.16 for Cl. The difference is 2.34, so the bond is ionic.
229
LESSON SUMMARY
LESSON SUMMARY
How can electronegativity be used to compare bonds?
KEY TERM
diatomic molecule
Electronegativity values can help you to compare and classify different bonds. If there is no difference in electronegativity between the two atoms, the bond is considered nonpolar covalent. The greater the electronegativity difference, the more polar the bond. The electrons in a polar bond tend to spend more time around the more electronegative atom. When the difference between the electronegativities of two bonded atoms is greater than about 2.1, the bond is considered ionic and one atom gives up an electron to the other atom, forming two ions.
Exercises
Reading Questions
Explain how electronegativity values help you to determine the polarity of a bond between two atoms.
How can you determine which atom in a covalent bond is partially positive?
Reason and Apply
Consider the following pairs of atoms. Place each set in order of increasing bond polarity. Describe the trend.
a. Li–F Na–F K–F Rb–F Cs–F b. Mg–O P–S N–F K–Cl Al–N Place a partial positive or a partial negative charge on each atom in the following pairs of atoms. Describe the trend.
H–B
H–C
H–N
H–O
H–F
Is hydrogen always partially positive when bonded to another atom? Explain.
Name three pairs of atoms with ionic bonds. For each pair, show the difference in electronegativity between the two atoms.
Name three pairs of atoms with polar covalent bonds. For each pair, show the difference in electronegativity between the two atoms.
Describe or draw what happens to the electrons in a polar covalent bond, a nonpolar bond, and an ionic bond.
What do we mean when we say that bonding is on a continuum?