LESSON 48: Protein Origami: Amino Acids and Proteins
THINK ABOUT IT
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The human body uses 20 amino acids. Of those 20 amino acids, about half can be produced by the body and aren’t necessary in our diets. The others cannot be produced by the body, so we need to get them from food. Sources of these amino acids—in the form of protein—include meat, eggs, grains, legumes, and dairy products.

Smelly molecules are small or medium-sized molecules that travel as a gas into your nose. Once in your nose, these molecules interact with receptor sites, which send a signal to your brain. Receptor sites are also built of atoms bonded together to create a “pocket” into which a specific smell molecule can fit.
What is a receptor site made of?
To answer this question, you will explore
Amino Acids
Proteins
Amino Acids
EXPLORING THE TOPIC
Amino Acids
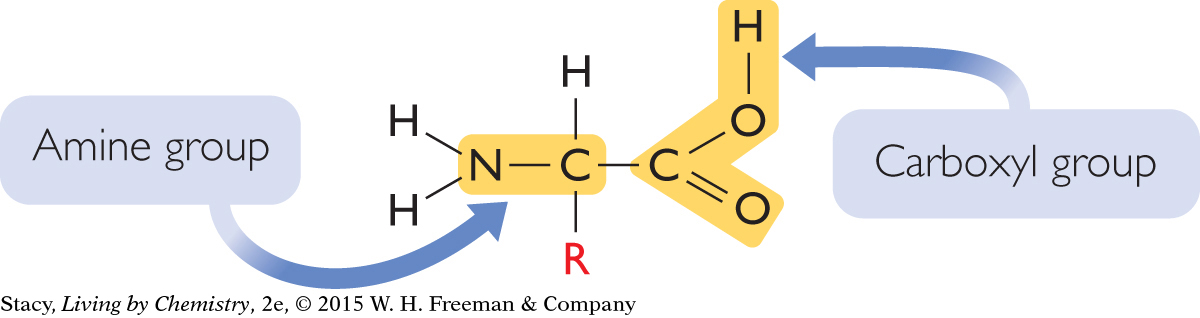
Amino acids are the building materials for many of the structures in the body, especially those related to the functioning of cells. They are important molecules in all living organisms. As the name implies, amino acids have two functional groups: an amine group and a carboxyl group. The general formula for an amino acid is C2H4NO2R, where R is simply a placeholder in the formula for a variety of possible groups of atoms. The groups consist of carbon and hydrogen, and sometimes nitrogen and/or oxygen.
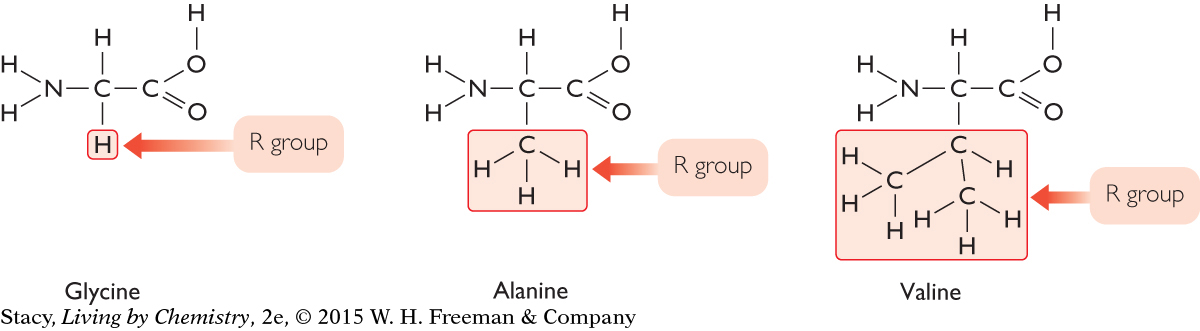
Here are structural formulas for three amino acids with the R groups highlighted. Notice that everything else about them is identical.
You might notice that the carbon atom in the center of the amino acid molecule has four different groups attached to it. Therefore, amino acids have mirror-image isomers and can be left-handed or right-handed molecules. There’s a trick to figuring out the handedness of an amino acid. The illustration shows you how.
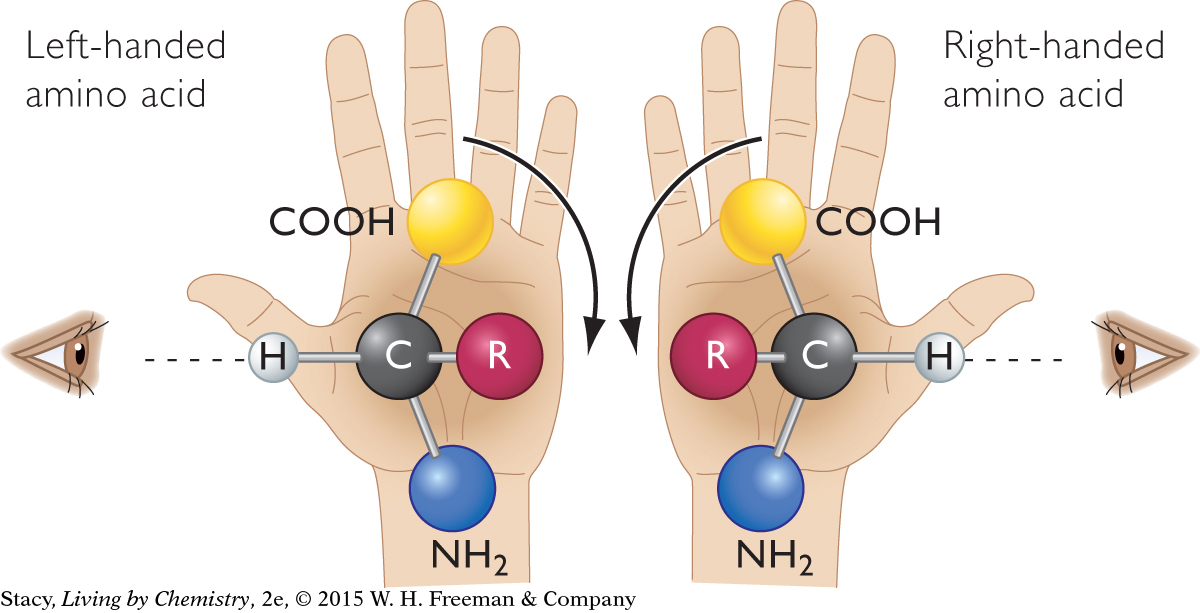
The human body uses only the left-handed amino acids, but both mirror images are available. How did life begin selecting only one of the two mirror images? This is a question of heated debate. Currently, no one has a good explanation.
There are 20 amino acids, with different R groups, that your body needs. Your body produces about half of these amino acids. You get the others as part of your diet. Six of the amino acids are listed in this illustration. These amino acids are placed into two categories: either hydrophobic (“water fearing”) or hydrophilic (“water loving”). Take a moment to determine the difference.
BIOLOGY CONNECTION
BIOLOGY
CONNECTION
The average human can distinguish among approximately 10,000 different odors. The number of receptor sites in the nose is estimated to be around 1000 by some researchers. This means that some smells must be stimulated by a combination of different receptors, much as a musical chord on a piano has a distinct sound.

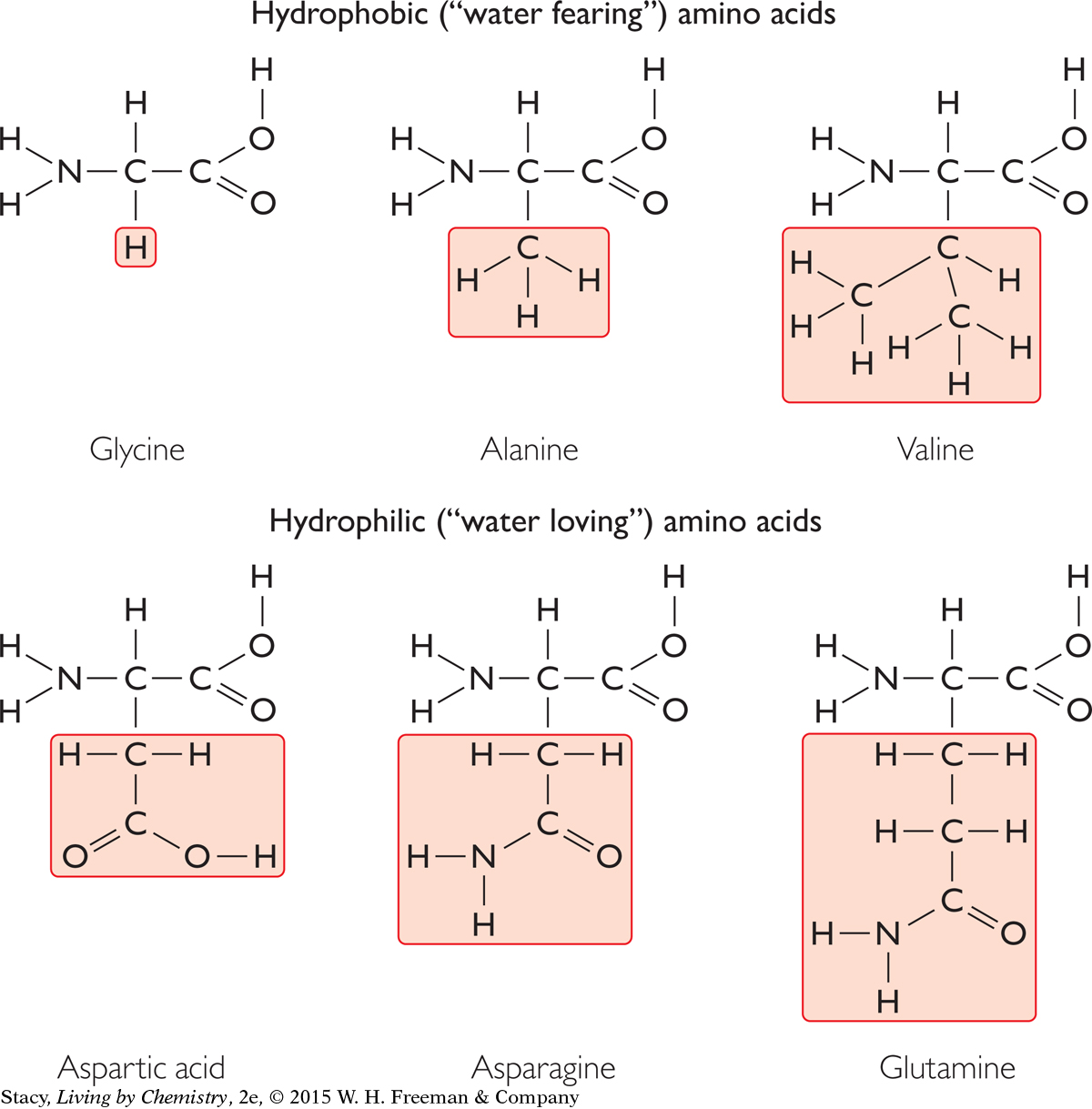
The R groups for the amino acids classified as hydrophobic contain only carbon and hydrogen atoms. These R groups are nonpolar. They are not attracted to water molecules. The R groups for the amino acids classified as hydrophilic contain atoms such as oxygen and nitrogen. These R groups are polar and are attracted to water molecules. As you will see, the nature of the R group is important when amino acids are linked to form proteins.
Proteins
Proteins
As shown in the next illustration, the carboxyl group of one amino acid can link to the amine group on another amino acid. The bond between two amino acids is called an amide bond, or a peptide bond. In the process, two hydrogen atoms and an oxygen atom break off from the amino acids and form a water molecule. In this way, it is possible to form long chains of amino acids called proteins. Because they are very large molecules, proteins are often called macromolecules.
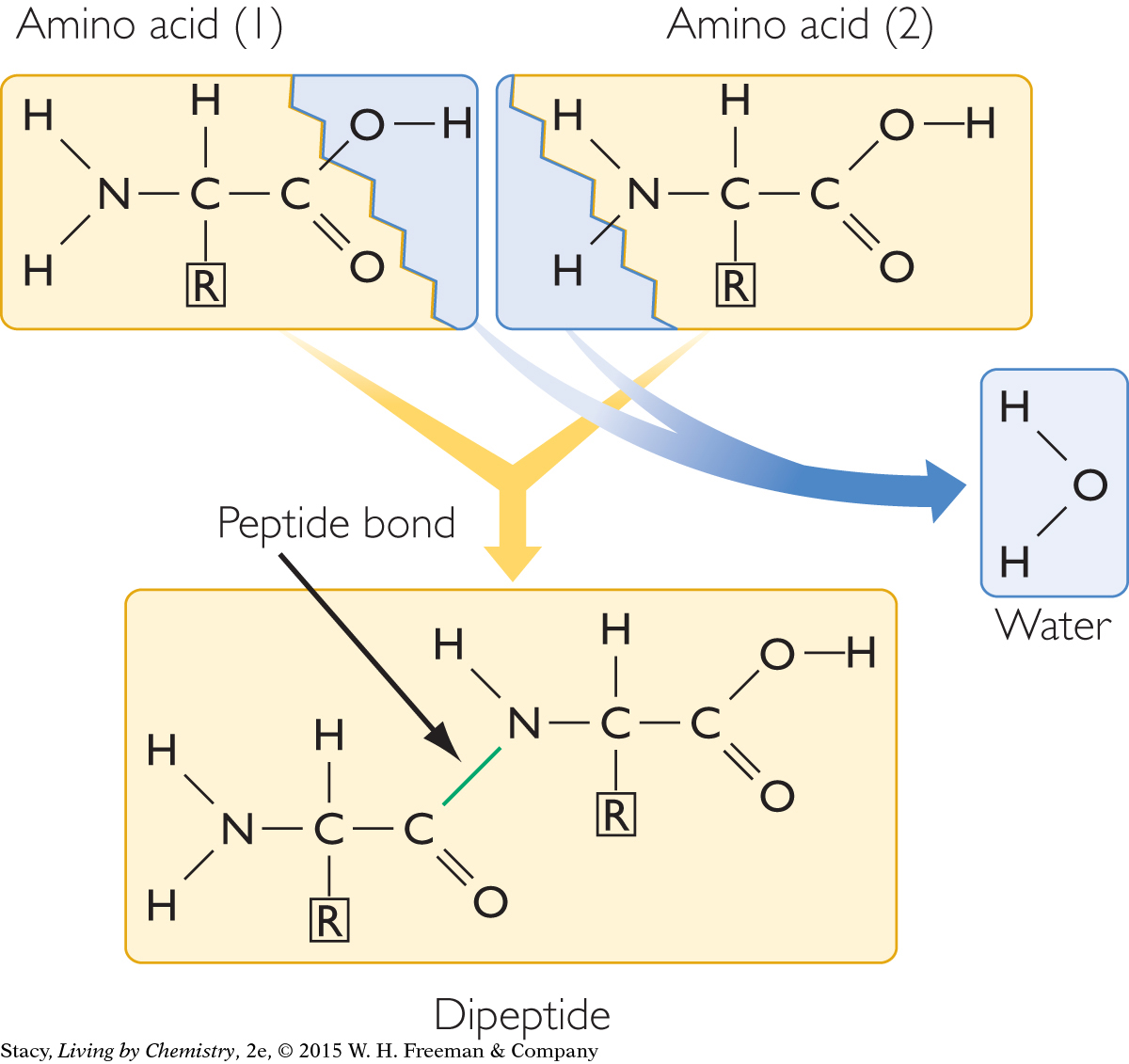
Proteins are essential in all living organisms. Protein molecules send messages throughout your body, they help the immune system fight off infections, and they are building blocks of many parts of your body, including organs, hair, and muscles. Proteins have a wide variety of structures and functions because many different sequences of amino acids are possible.

As the chain of amino acids forms, it twists and bends. In the photograph, each bead represents an amino acid. The entire chain of beads represents a protein molecule. Notice that amino acids can repeat.
The R groups on each amino acid play a major role in determining the resulting structure of the protein. The hydrophobic R groups tend to cluster in the center of the molecule. The hydrophilic R groups tend to be on the outside of the molecule, near the watery environment.
Smell receptor sites are protein molecules that have a pocket that fits a particular smell molecule. When the smell molecule attaches to the pocket, it changes the structure of the protein just a bit. This small change is enough to affect a neighboring protein, which triggers a signal to the brain.
LESSON SUMMARY
LESSON SUMMARY
KEY TERMS
amino acid
peptide bond (amide bond)
protein
What is a receptor site made of?
There are 20 different amino acid molecules that are essential building blocks of the human body. Amino acids have both a carboxyl functional group and an amine functional group. Amino acids all have mirror-image isomers, but only left-handed amino acid molecules are used by the human body. The carboxyl group on one amino acid can link with the amine group on another amino acid to form a peptide bond. In this way, a long chain of amino acids called a protein forms. A large variety of protein molecules exists because the amino acids can be linked in a variety of sequences. Some amino acids in a protein chain are polar and others are nonpolar. This affects the folding of a chain of amino acids in the protein. Smell receptor sites are composed of proteins.
Exercises
Reading Questions
What is an amino acid?
What is a protein?
Reason and Apply
Draw a diagram to show how glycine and alanine combine to form a peptide bond.
Imagine a protein molecule composed of 200 glycine molecules and 200 aspartic acid molecules. Describe how the protein is folded. What is on the inside and what is on the outside?
Use the Internet to research amino acids. Describe three different sources of amino acids in a person’s diet. Describe how forming a peptide bond is like forming an ester from an acid and an alcohol.