Review Introduction
140

UNIT
1
Alchemy | REVIEW
Matter is anything that has substance and takes up space. Mass is a measure of the substance of matter. Volume is a measure of the space that matter takes up. The density of a substance is the mass per unit of volume.
Everything on the planet is composed of elements or compounds made from combinations of these elements. The elements are organized according to their properties and atomic structure into a chart called the periodic table. The periodic table holds a wealth of information about the elements and their atoms. Elements that are located in the same column of the periodic table tend to have similar behavior and reactivity. The noble gases are very stable elements with extremely low reactivity.
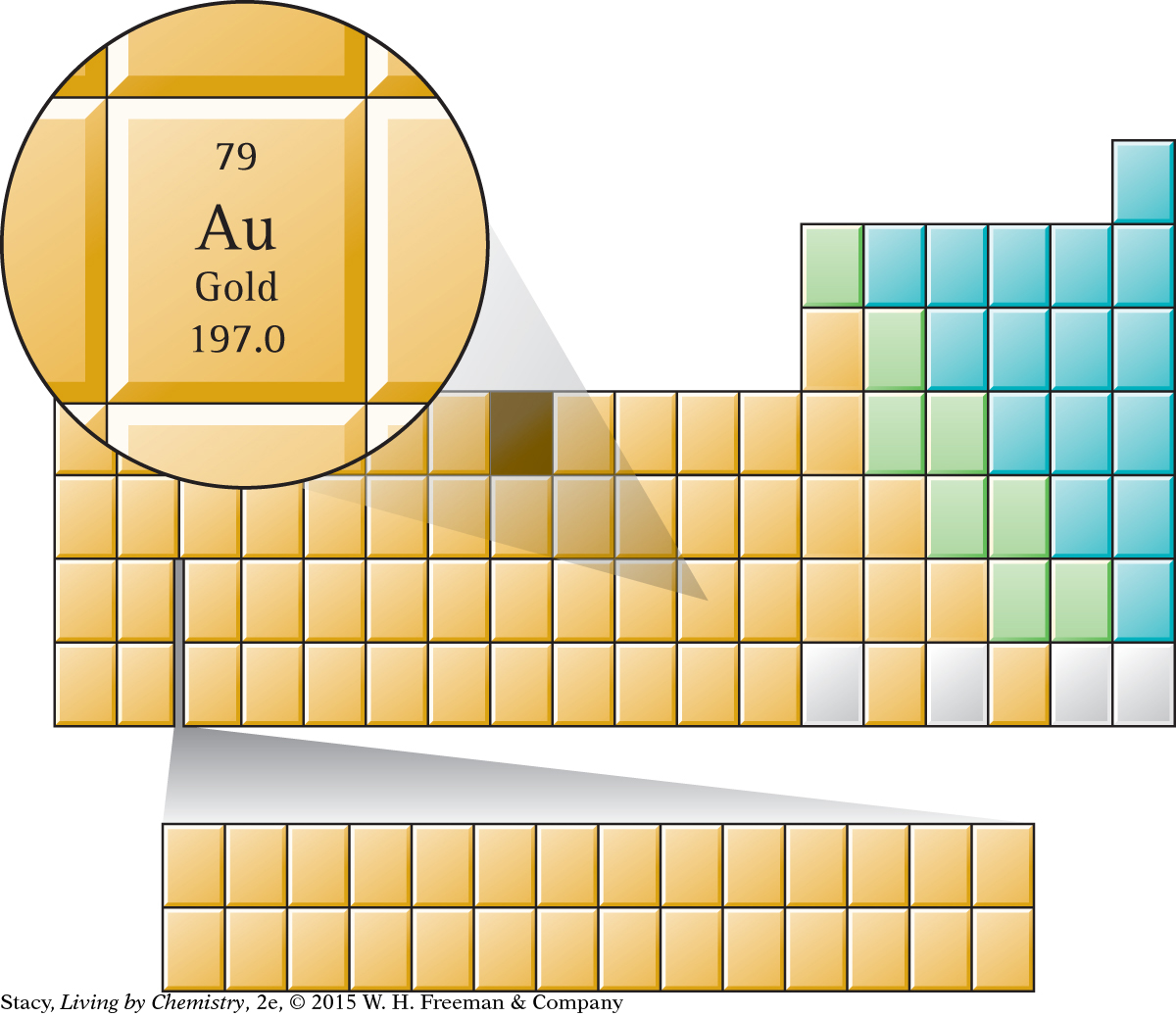
Matter is composed of individual building blocks called atoms. Atoms are much too small to be seen, so experimental evidence has led to various models of the atom. In the center of each atom is a dense nucleus consisting of protons and neutrons. Most of the mass of an atom is located in its nucleus. Most atoms have more neutrons than protons in their nuclei, which keeps the nucleus more stable.
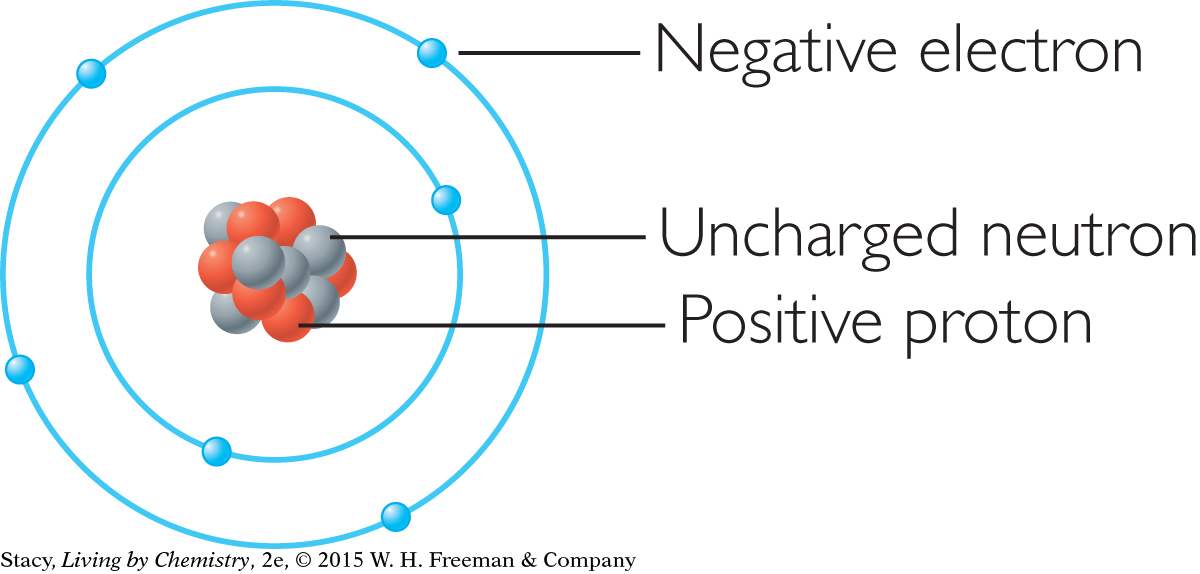
Electrons are located at specific distances from the nucleus, called shells. The electrons in the outermost shell of an atom are called its valence electrons and are key to its behavior. Atoms will sometimes lose or gain valence electrons, forming ions with positive and negative charges, and achieving the electron arrangements of the noble gases.
A compound is a substance that contains atoms of more than one type of element, bonded together. There are millions of compounds in existence and many yet to be discovered.
141
A bond is an attractive force that keeps atoms connected to one another. The attraction occurs between the positively charged nuclei and the negatively charged electrons in different atoms. Usually the valence electrons are involved in bonding.
There are four main models for bonding: ionic, molecular covalent, metallic, and network covalent. These models can be used to explain difference in solubility and conductivity.