STANDARDIZED TEST PREPARATION
Multiple Choice
Choose the best answer.
Question 1
1. Which option has only units of pressure?
atm, lb/in2, in Hg
lb/in2, in Hg, mL/K
in Hg, mL/K, atm
mL/K, atm, lb/in2
350
Question 2
2. A cylindrical rain gauge contains a volume of 6 mL for a height of 2 cm of rain. What is the volume of rain for a height of 9 cm in this rain gauge?
9 mL
18 mL
27 mL
54 mL
Use the graph below for Exercises 3 and 4.
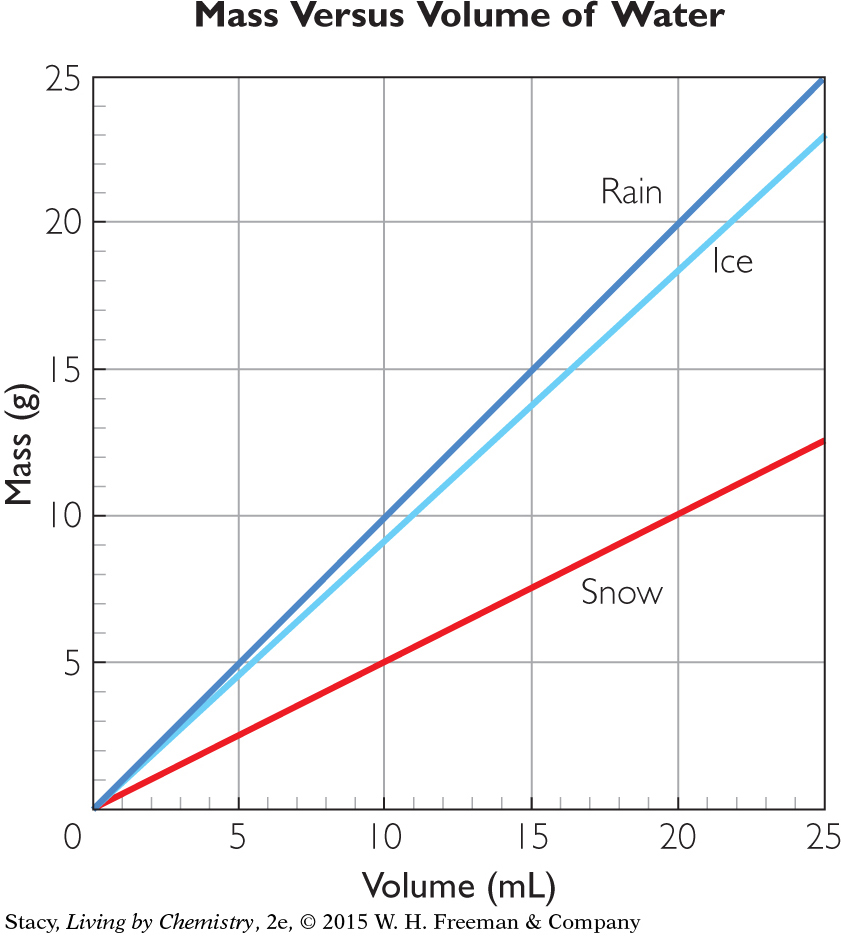
Question 3
3. For equal volume samples of snow, ice, and rain, which statement is correct in describing the mass of the samples?
The masses of each will be the same because in each case, the substance is water, H2O.
The mass of snow will be smallest because the snow has the highest density.
The mass of the ice will be smallest because the ice has the lowest density.
The mass of the rain will be largest because the rain has the highest density.
Question 4
4. What is the volume of 15.0 g of ice?
7.5 mL
13.8 mL
15.0 mL
16.3 mL
Question 5
5. The density of solid nitrogen is 1.026 g/cm3 and the density of nitrogen gas at STP is 0.00125 g/cm3. What is the volume of gas produced when 50 mL of solid nitrogen sublimes?
39 L
41 L
61 L
64 L
Question 6
6. The average temperature at the top of Mt. Denali in Alaska can drop below –50 °F. What is this temperature in Kelvins?
125 K
263 K
227 K
283 K
Question 7
7. Which of the following correctly describes the movement of gas particles according to the kinetic theory of gases?
All gas particles move with the same speed at a given temperature.
All gas particles move in curved lines in random directions.
The average speed of gas particles increases with decreasing temperature.
Gas particles are in constant motion at temperatures above 0 K.
Question 8
8. Imagine that a gas sample is in a cylinder with a piston. Assuming that the pressure and number of molecules stays the same, what will happen to the volume of the gas if the temperature increases?
The volume decreases because the particles are closer together.
The volume decreases because the particles have collided less.
The volume increases because the particles are moving faster.
The volume increases because the particles are less attracted to each other.
Question 9
9. A balloon is filled with air to a volume of 1.35 L at a temperature of 22 °C. The balloon is taken to a birthday picnic on a sunny day. After several hours in the Sun, the air inside of the balloon was warmed to 32 °C. What is the new volume of the balloon?
2.0 L
1.3 L
1.4 L
0.93 L
Question 10
10. What phase change occurs when a cloud forms from water vapor?
Evaporation
Condensation
Melting
Freezing
351
Question 11
11. A weather balloon expands as it travels to a higher altitude. Which of the following correctly describes the gas particles inside of the balloon?
There is more space between the gas particles inside the balloon because the atmospheric pressure is less at higher altitudes.
There is more space between the gas particles inside the balloon because the atmospheric pressure is greater at higher altitudes.
There are more collisions between the gas particles inside of the balloon due to the lower atmospheric pressure.
The average speed of the gas particles inside the balloon increases due to the decrease in temperature at higher altitudes.
Question 12
12. A 0.75 L balloon has a pressure of 1.0 atm. The balloon is placed inside a vacuum chamber, in which air outside the balloon can be removed. After air has been removed from the chamber, the pressure inside the balloon is reduced to 0.50 atm. The temperature remains unchanged. What is the new volume of the balloon?
0.38 L
0.50 L
0.67 L
1.5 L
Question 13
13. Which hypothesis best illustrates the particle view of what occurs when solid carbon dioxide sublimes?
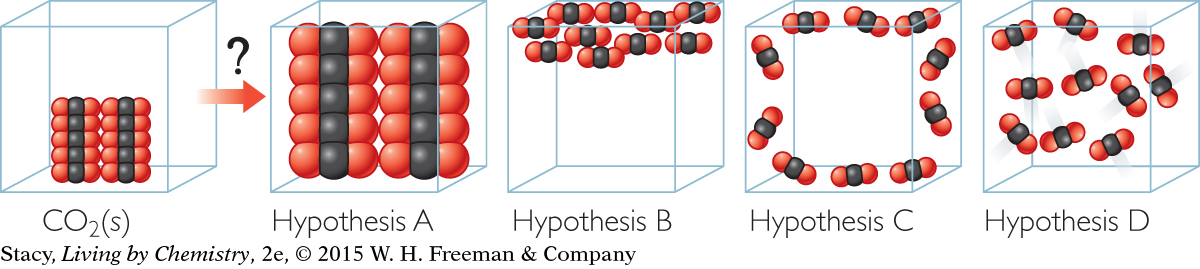
Hypothesis A. The gas molecules individually expand when carbon dioxide sublimes.
Hypothesis B. The gas molecules float to the top of the container when carbon dioxide sublimes.
Hypothesis C. The gas molecules move to the surfaces of the container when carbon dioxide sublimes.
Hypothesis D. The gas molecules move relatively far apart from one another when carbon dioxide sublimes.
Question 14
14. An 8.5 L scuba tank is filled with air to a pressure of 150 atm at 26 °C. The scuba tank is cooled to a temperature of 15 °C. What is the pressure inside of the tank?
17 atm
86 atm
140 atm
160 atm
Question 15
15. Which of the following statements correctly describes the relationship between gas measurements?
When temperature and the amount of gas do not change, the pressure and volume of the gas are inversely proportional.
When pressure and the amount of gas do not change, the volume and temperature are inversely proportional.
When the amount of gas and volume do not change, the pressure and temperature are inversely proportional.
When the temperature and pressure do not change, the number of particles and volume are inversely proportional.
Question 16
16. A weather balloon is filled with helium gas to a volume of 7500 L at sea level where the pressure is 1 atm and the temperature is 24 °C. The balloon travels to the top of the stratosphere where the temperature is –15 °C and the pressure is 0.001 atm. What is the volume of the balloon while at this altitude?
4.7 × 106 L
6.5 × 106 L
8.6 × 106 L
1.2 × 107 L
352
Question 17
17. Suppose you have two balloons, one filled with helium, He, and one filled with argon, Ar. Each balloon has a volume of 22.4 L at STP. Which of the following correctly describes these samples?
The helium balloon contains fewer gas molecules because the atomic number of helium is less than that of argon.
The helium balloon contains fewer gas molecules because the mass number of helium is less than that of argon.
Each balloon contains the same number of gas particles because the balloons are the same volume, at the same temperature and pressure.
Each balloon contains a different number of gas particles because each balloon is filled with a different gas.
Question 18
18. Complete the data set for a sample of gas.
| Pressure | Volume | Temperature | Moles |
| 0.75 atm | 11.2 L | 300 K | ? |
0.34 mol
0.50 mol
2.93 mol
244 mol
Question 19
19. How many moles of N2 gas are there in a container that holds 11.2 L at 0 °C and 1 atm?
0.25 mol
0.50 mol
1.0 mol
2.0 mol
Question 20
20. Find the pressure of 2.5 mol of gas if the gas temperature is 32.0 °C and the gas volume is 67.2 L.
10.2 atm
1.07 atm
0.930 atm
0.0976 atm