Review Introduction

UNIT
6
Showtime | REVIEW
Many chemical processes are reversible. These processes do not convert all of the starting substances to products. Instead, the result is an equilibrium mixture of starting substances and products. In reversible chemical processes, the forward and reverse processes continue. When the forward and reverse processes occur at equal rates, these systems are said to be at equilibrium. Reversible processes are written with a double arrow to show that the forward and reverse processes occur at the same time.
Common equilibrium mixtures involve dissociation processes in which a starting substance breaks apart into two or more pieces. The back-and-forth evaporation and condensation of water on the planet is a form of phase equilibrium. Salts dissolve in water and dissociate into cations and anions, and the cations and anions combine to re-form the salt. Weak acids in water dissociate into H+ and an anion, and they recombine to form the acid molecule. Molecules in the body attach and detach from receptor sites. In all of these cases, there is a dynamic balance between the starting substances and products.
There is a mathematical relationship between the products and starting substances in an equilibrium mixture. The equilibrium constant K and the equilibrium-constant equation can be used to solve problems involving systems at equilibrium.
aA + bB ⇋ cC + dD
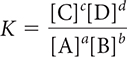
In general, a large value of K indicates that products are favored in the equilibrium mixture, and a small value of K indicates that starting substances are favored.
Le Châtelier’s principle states that when conditions are changed for an equilibrium mixture, the system will respond by reducing the effect of the change. When conditions such as temperature, pressure, and concentration are changed in a system, the system regains equilibrium by rebalancing the concentrations of starting substances and products.