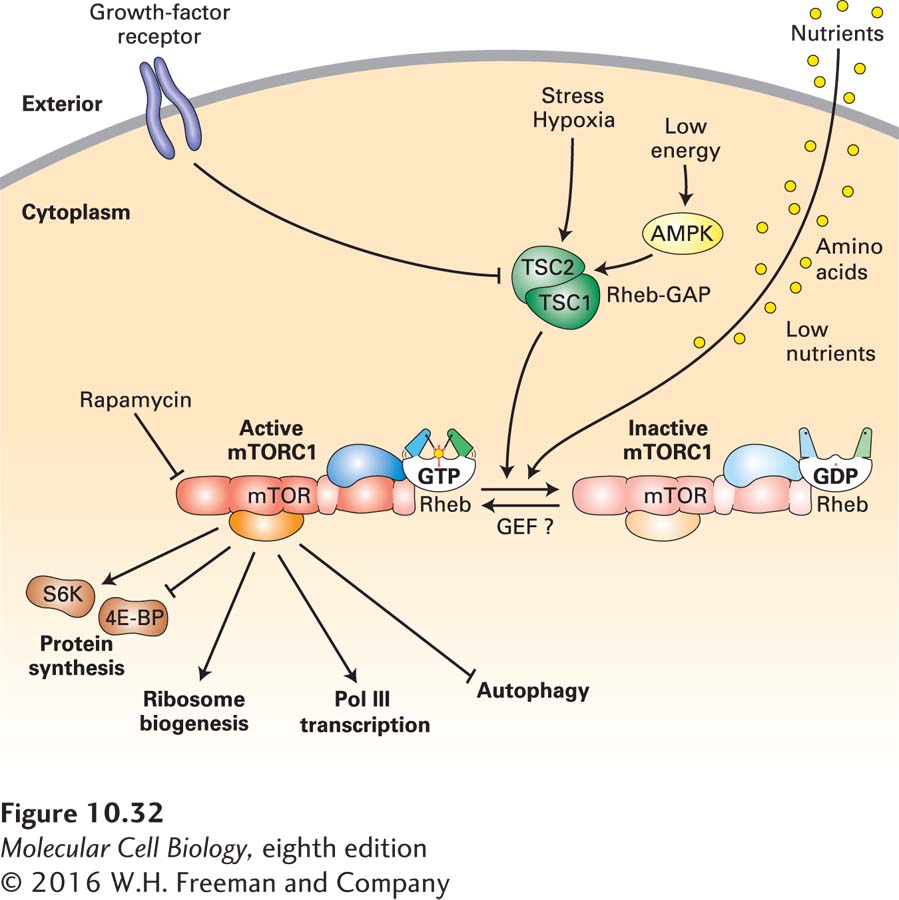
FIGURE 10- b- b- b- b- l- h- E-